Abstract
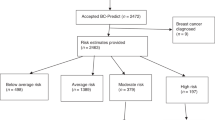
It has been shown in several studies that antihormonal compounds can offer effective prophylactic treatment to prevent breast cancer. In view of the low participation rates in chemoprevention trials, the purpose of this study was to identify the characteristics of women taking part in a population-based mammography screening program who wished to obtain information about the risk of breast cancer and then participate in the the International Breast Cancer Intervention Study II (IBIS-II) trial, a randomized double-blind controlled chemoprevention trial comparing anastrozole with placebo. A paper-based survey was conducted in a population-based mammography screening program in Germany between 2007 and 2009. All women who met the criteria for the mammography screening program were invited to complete a questionnaire. A total of 2,524 women completed the questionnaire, and 17.7% (n = 446) met the eligibility criteria for the IBIS-II trial after risk assessment. The women who wished to receive further information about chemoprevention were significantly younger (P < 0.01) and had significantly more children (P = 0.03) and significantly more relatives with breast cancer (P < 0.001). There were no significant differences between the participants with regard to body mass index or hormone replacement therapy. Normal mammographic findings at screening were the main reason (42%) for declining to participate in the IBIS-II trial or attend risk counseling. The ultimate rate of recruitment to the IBIS-II trial was very low (three women). Offering chemoprevention to women within a mammography screening unit as part of a paper-based survey resulted in low participation rates for both, the survey and the final participation in the IBIS-II trial. More individualized approaches and communication of breast cancer risk at the time of the risk assessment might be helpful to increase the participation and the understanding of chemopreventive approaches.

Similar content being viewed by others
References
Cuzick J, Forbes J, Edwards R et al (2002) First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet 360:817–824
Fisher B, Costantino JP, Wickerham DL et al (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90:1371–1388
Powles T, Eeles R, Ashley S et al (1998) Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet 352:98–101
Veronesi U, Maisonneuve P, Sacchini V, Rotmensz N, Boyle P (2002) Tamoxifen for breast cancer among hysterectomised women. Lancet 359:1122–1124
Cuzick J, Powles T, Veronesi U et al (2003) Overview of the main outcomes in breast-cancer prevention trials. Lancet 361:296–300
Cauley JA, Norton L, Lippman ME et al (2001) Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat 65:125–134
Vogel VG, Costantino JP, Wickerham DL et al (2006) Effects of tamoxifen vs. raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295:2727–2741
Goss PE, Ingle JN, Martino S et al (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349:1793–1802
Baum M, Budzar AU, Cuzick J et al (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139
Thurlimann B, Keshaviah A, Coates AS et al (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353:2747–2757
Coombes RC, Hall E, Gibson LJ et al (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081–1092
Jakesz R, Jonat W, Gnant M et al (2005) Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366:455–462
Mouridsen H, Giobbie-Hurder A, Goldhirsch A et al (2009) Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med 361:766–776
Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 9:45–53
Coombes RC, Kilburn LS, Snowdon CF et al (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369:559–570
Cuzick J (2003) Aromatase inhibitors in prevention—data from the ATAC (Arimidex, tamoxifen alone or in combination) trial and the design of IBIS-II (the second International Breast Cancer Intervention Study). Recent Results Cancer Res 163:96–103 (discussion 264–266)
Goss PE, Richardson H, Chlebowski R et al (2007) National Cancer Institute of Canada Clinical Trials Group MAP.3 Trial: evaluation of exemestane to prevent breast cancer in postmenopausal women. Clin Breast Cancer 7:895–900
Editorial (2007) NCI and the STELLAR trial. Lancet 369:2134
Dunn BK, Ryan A (2009) Phase 3 trials of aromatase inhibitors for breast cancer prevention: following in the path of the selective estrogen receptor modulators. Ann N Y Acad Sci 1155:141–161
Powles TJ (2002) Breast cancer prevention. Oncologist 7:60–64
Vescia S, von Minckwitz G, Loibl S et al (2008) The GISS Trial: a pilot phase randomized prevention trial of screening plus goserelin plus ibandronate, versus screening alone in premenopausal women at increased risk of breast cancer (abstract). Eur J Cancer 6:72
von Minckwitz GPB, Hofmann K et al (2002) Prevention with goserelin and ibandronate in premenopausal women with familial breast cancer risk: first experiences of the GISS study (abstract). Arch Gynecol Obstet 267(Suppl 1):S52
Evans D, Lalloo F, Shenton A, Boggis C, Howell A (2001) Uptake of screening and prevention in women at very high risk of breast cancer. Lancet 358(9285):889–890
Schulz-Wendtland R, Becker N, Bock K, Anders K, Bautz W (2007) Mammography screening. Radiologe 47:359–369 (quiz 70)
Kooperationsgemeinschaft-Mammographie (2009) Evaluationsbericht 2005–2007: Ergebnisse des Mammographie-Screening-Programms in Deutschland 2009. www.mammo-programm.de/fachinformationen/evaluation.php
Tyrer J, Duffy SW, Cuzick J (2004) A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 23:1111–1130
Fasching PA, von Minckwitz G, Fischer T et al (2007) The impact of breast cancer awareness and socioeconomic status on willingness to receive breast cancer prevention drugs. Breast Cancer Res Treat 101:95–104
The Million Women Study Collaborative Group (1999) The Million Women Study: design and characteristics of the study population. The Million Women Study Collaborative Group. Breast Cancer Res 1:73–80
The Women’s Health Initiative Study Group (1998) Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials 19:61–109
Yeomans-Kinney A, Vernon SW, Frankowski RF, Weber DM, Bitsura JM, Vogel VG (1995) Factors related to enrollment in the breast cancer prevention trial at a comprehensive cancer center during the first year of recruitment. Cancer 76:46–56
Rossouw JE, Anderson GL, Prentice RL et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333
Majumdar SR, Almasi EA, Stafford RS (2004) Promotion and prescribing of hormone therapy after report of harm by the Women’s Health Initiative. JAMA 292:1983–1988
Beral V (2003) Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362:419–427
Chlebowski RT, Kuller LH, Prentice RL et al (2009) Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med 360:573–587
Gail MH, Brinton LA, Byar DP et al (1989) Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 81:1879–1886
Claus EB, Risch N, Thompson WD (1994) Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 73:643–651
Parmigiani G, Berry D, Aguilar O (1998) Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet 62:145–158
Antoniou AC, Pharoah PP, Smith P, Easton DF (2004) The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer 91:1580–1590
Fasching PA, Bani MR, Nestle-Kramling C, Goecke TC, Niederacher D, Beckmann MW, Lux MP (2007) Evaluation of mathematical models for breast cancer risk assessment in routine clinical use. Eur J Cancer Prev 16:216–224
Boyd NF, Guo H, Martin LJ et al (2007) Mammographic density and the risk and detection of breast cancer. N Engl J Med 356:227–236
Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K (2008) Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med 148(5):337–347
Chen J, Pee D, Ayyagari R, Graubard B, Schairer C, Byrne C, Benichou J, Gail MH (2006) Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst 98(17):1215–1226
Cummings SR, Tice JA, Bauer S, Browner WS, Cuzick J, Ziv E, Vogel V, Shepherd J, Vachon C, Smith-Bindman R et al (2009) Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst 101(6):384–398
Acknowledgements
This project was partly sponsored by AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Additional information
Christian R. Loehberg and Sebastian M. Jud contributed equally to this study.
Rights and permissions
About this article
Cite this article
Loehberg, C.R., Jud, S.M., Haeberle, L. et al. Breast cancer risk assessment in a mammography screening program and participation in the IBIS-II chemoprevention trial. Breast Cancer Res Treat 121, 101–110 (2010). https://doi.org/10.1007/s10549-010-0845-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0845-8