Abstract
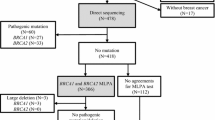
Large genomic rearrangements (LGR) represent substantial proportion of pathogenic mutations in the BRCA1 gene, whereas the frequency of rearrangements in the BRCA2 gene is low in many populations. We screened for LGRs in BRCA1 and BRCA2 genes by multiplex ligation-dependent probe amplification (MLPA) in 521 unrelated patients negative for BRCA1/2 point mutations selected from 655 Czech high-risk breast and/or ovarian cancer patients. Besides long range PCR, a chromosome 17-specific oligonucleotide-based array comparative genomic hybridization (aCGH) was used for accurate location of deletions. We identified 14 patients carrying 8 different LGRs in BRCA1 that accounted for 12.3% of all pathogenic BRCA1 mutations. No LGRs were detected in the BRCA2 gene. In a subgroup of 239 patients from high-risk families, we found 12 LGRs (5.0%), whereas two LGRs were revealed in a subgroup of 282 non-familial cancer cases (0.7%). Five LGRs (deletion of exons 1–17, 5–10, 13–19, 18–22 and 21–24) were novel; two LGRs (deletion of exons 5–14 and 21–22) belong to the already described Czech-specific mutations; one LGR (deletion of exons 1–2) was reported from several countries. The deletions of exons 1–17 and 5–14, identified each in four families, represented Czech founder mutations. The present study indicates that screening for LGRs in BRCA1 should include patients from breast or ovarian cancer families as well as high-risk patients with non-familial cancer, in particular cases with early-onset breast or ovarian cancer. On the contrary, our analyses do not support the need to screen for LGRs in the BRCA2 gene. Implementation of chromosome-specific aCGH could markedly facilitate the design of primers for amplification and sequence analysis of junction fragments, especially in deletions overlapping gene boundaries.




Similar content being viewed by others
References
Anglian Breast Cancer Study Group (2000) Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer 83:1301–1308
Malone KE, Daling JR, Doody DR, Hsu L, Bernstein L, Coates RJ, Marchbanks PA, Simon MS, McDonald JA, Norman SA, Strom BL, Burkman RT, Ursin G, Deapen D, Weiss LK, Folger S, Madeoy JJ, Friedrichsen DM, Suter NM, Humphrey MC, Spirtas R, Ostrander EA (2006) Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res 66:8297–8308
Mateju M, Stribrna J, Zikan M, Kleibl Z, Janatova M, Kormunda S, Novotny J, Soucek P, Petruzelka L, Pohlreich P (2010) Population-based study of BRCA1/2 mutations: family history based criteria identify minority of mutation carriers. Neoplasma 57(4)
Hogervorst FB, Nederlof PM, Gille JJ, McElgunn CJ, Grippeling M, Pruntel R, Regnerus R, van Welsem T, van Spaendonk R, Menko FH, Kluijt I, Dommering C, Verhoef S, Schouten JP, van’t Veer LJ, Pals G (2003) Large genomic deletions and duplications in the BRCA1 gene identified by a novel quantitative method. Cancer Res 63:1449–1453
Mazoyer S (2005) Genomic rearrangements in the BRCA1 and BRCA2 genes. Hum Mutat 25:415–422
de la Hoya M, Gutierrez-Enriquez S, Velasco E, Osorio A, Sanchez de Abajo A, Vega A, Salazar R, Esteban E, Llort G, Gonzalez-Sarmiento R, Carracedo A, Benitez J, Miner C, Diez O, Diaz-Rubio E, Caldes T (2006) Genomic rearrangements at the BRCA1 locus in Spanish families with breast/ovarian cancer. Clin Chem 52:1480–1485
Engert S, Wappenschmidt B, Betz B, Kast K, Kutsche M, Hellebrand H, Goecke TO, Kiechle M, Niederacher D, Schmutzler RK, Meindl A (2008) MLPA screening in the BRCA1 gene from 1,506 German hereditary breast cancer cases: novel deletions, frequent involvement of exon 17, and occurrence in single early-onset cases. Hum Mutat 29:948–958
Gad S, Caux-Moncoutier V, Pages-Berhouet S, Gauthier-Villars M, Coupier I, Pujol P, Frenay M, Gilbert B, Maugard C, Bignon YJ, Chevrier A, Rossi A, Fricker JP, Nguyen TD, Demange L, Aurias A, Bensimon A, Stoppa-Lyonnet D (2002) Significant contribution of large BRCA1 gene rearrangements in 120 French breast and ovarian cancer families. Oncogene 21:6841–6847
Hansen TV, Jonson L, Albrechtsen A, Andersen MK, Ejlertsen B, Nielsen FC (2009) Large BRCA1 and BRCA2 genomic rearrangements in Danish high risk breast-ovarian cancer families. Breast Cancer Res Treat 115:315–323
Puget N, Stoppa-Lyonnet D, Sinilnikova OM, Pages S, Lynch HT, Lenoir GM, Mazoyer S (1999) Screening for germ-line rearrangements and regulatory mutations in BRCA1 led to the identification of four new deletions. Cancer Res 59:455–461
Petrij-Bosch A, Peelen T, van Vliet M, van Eijk R, Olmer R, Drusedau M, Hogervorst FB, Hageman S, Arts PJ, Ligtenberg MJ, Meijers-Heijboer H, Klijn JG, Vasen HF, Cornelisse CJ, ‘t Veer LJ, Bakker E, van Ommen GJ, Devilee P (1997) BRCA1 genomic deletions are major founder mutations in Dutch breast cancer patients. Nat Genet 17:341–345
Agata S, Viel A, Della PL, Cortesi L, Fersini G, Callegaro M, Dalla PM, Dolcetti R, Federico M, Venuta S, Miolo G, D’Andrea E, Montagna M (2006) Prevalence of BRCA1 genomic rearrangements in a large cohort of Italian breast and breast/ovarian cancer families without detectable BRCA1 and BRCA2 point mutations. Genes Chromosomes Cancer 45:791–797
Smith TM, Lee MK, Szabo CI, Jerome N, McEuen M, Taylor M, Hood L, King MC (1996) Complete genomic sequence and analysis of 117 kb of human DNA containing the gene BRCA1. Genome Res 6:1029–1049
Puget N, Gad S, Perrin-Vidoz L, Sinilnikova OM, Stoppa-Lyonnet D, Lenoir GM, Mazoyer S (2002) Distinct BRCA1 rearrangements involving the BRCA1 pseudogene suggest the existence of a recombination hot spot. Am J Hum Genet 70:858–865
Buffone A, Capalbo C, Ricevuto E, Sidoni T, Ottini L, Falchetti M, Cortesi E, Marchetti P, Scambia G, Tomao S, Rinaldi C, Zani M, Ferraro S, Frati L, Screpanti I, Gulino A, Giannini G (2007) Prevalence of BRCA1 and BRCA2 genomic rearrangements in a cohort of consecutive Italian breast and/or ovarian cancer families. Breast Cancer Res Treat 106:289–296
Preisler-Adams S, Schonbuchner I, Fiebig B, Welling B, Dworniczak B, Weber BH (2006) Gross rearrangements in BRCA1 but not BRCA2 play a notable role in predisposition to breast and ovarian cancer in high-risk families of German origin. Cancer Genet Cytogenet 168:44–49
Pylkas K, Erkko H, Nikkila J, Solyom S, Winqvist R (2008) Analysis of large deletions in BRCA1, BRCA2 and PALB2 genes in Finnish breast and ovarian cancer families. BMC Cancer 8:146
Staaf J, Torngren T, Rambech E, Johansson U, Persson C, Sellberg G, Tellhed L, Nilbert M, Borg A (2008) Detection and precise mapping of germline rearrangements in BRCA1, BRCA2, MSH2, and MLH1 using zoom-in array comparative genomic hybridization (aCGH). Hum Mutat 29:555–564
Agata S, Dalla PM, Callegaro M, Scaini MC, Menin C, Ghiotto C, Nicoletto O, Zavagno G, Chieco-Bianchi L, D’Andrea E, Montagna M (2005) Large genomic deletions inactivate the BRCA2 gene in breast cancer families. J Med Genet 42:e64
Gutierrez-Enriquez S, de la Hoya M, Martinez-Bouzas C, Sanchez dA, Cajal T, Llort G, Blanco I, Beristain E, Diaz-Rubio E, Alonso C, Tejada MI, Caldes T, Diez O (2007) Screening for large rearrangements of the BRCA2 gene in Spanish families with breast/ovarian cancer. Breast Cancer Res Treat 103:103–107
Tournier I, Paillerets BB, Sobol H, Stoppa-Lyonnet D, Lidereau R, Barrois M, Mazoyer S, Coulet F, Hardouin A, Chompret A, Lortholary A, Chappuis P, Bourdon V, Bonadona V, Maugard C, Gilbert B, Nogues C, Frebourg T, Tosi M (2004) Significant contribution of germline BRCA2 rearrangements in male breast cancer families. Cancer Res 64:8143–8147
Vasickova P, Machackova E, Lukesova M, Damborsky J, Horky O, Pavlu H, Kuklova J, Kosinova V, Navratilova M, Foretova L (2007) High occurrence of BRCA1 intragenic rearrangements in hereditary breast and ovarian cancer syndrome in the Czech Republic. BMC Med Genet 8:32
Zikan M, Pohlreich P, Stribrna J, Kleibl Z, Cibula D (2008) Novel complex genomic rearrangement of the BRCA1 gene. Mutat Res 637:205–208
Bartonkova H, Foretová L, Helmichová E, Kalábová R, Kleibl Z, Konopásek B, Krutílková V, Macháčková E, Novotný J, Petráková K, Petruželka L, Plevová P, Pohlreich P, Rob L, Skovajsová M, Veselý J, Žaloudík J (2003) Doporučené zásady péče o nemocné s nádory prsu a vaječníků a zdravé osoby se zárodečnými mutacemi genů BRCA1 nebo BRCA2 [Recommendations for care of patients with breast and ovarian cancer and healthy individuals with germline mutations in BRCA1 or BRCA2 gene]. Klin Onkol 16:28–34
Plevová P, Novotný J, Petráková K, Palácová M, Kalábová R, Schneiderová M, Foretová L (2009) Syndrom hereditárního karcinomu prsu a ovarií [Hereditary breast and ovarian cancer syndrome]. Klin Onkol 22(Suppl):S8–11
Pohlreich P, Stribrna J, Kleibl Z, Zikan M, Kalbacova R, Petruzelka L, Konopasek B (2003) Mutations of the BRCA1 gene in hereditary breast and ovarian cancer in the Czech Republic. Med Princ Pract 12:23–29
Pohlreich P, Zikan M, Stribrna J, Kleibl Z, Janatova M, Kotlas J, Zidovska J, Novotny J, Petruzelka L, Szabo C, Matous B (2005) High proportion of recurrent germline mutations in the BRCA1 gene in breast and ovarian cancer patients from the Prague area. Breast Cancer Res 7:R728–R736
Parmigiani G, Berry D, Aguilar O (1998) Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet 62:145–158
Workman C, Jensen LJ, Jarmer H, Berka R, Gautier L, Nielser HB, Saxild HH, Nielsen C, Brunak S, Knudsen S (2002) A new non-linear normalization method for reducing variability in DNA microarray experiments. Genome Biol 3: research0048.1–16
Olshen AB, Venkatraman ES, Lucito R, Wigler M (2004) Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics 5:557–572
den Dunnen JT, Antonarakis SE (2000) Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 15:7–12
International Human Genome Sequencing Consortium (2004) Finishing the euchromatic sequence of the human genome. Nature 431:931–945
Soukupova J, Dundr P, Kleibl Z, Pohlreich P (2008) Contribution of mutations in ATM to breast cancer development in the Czech population. Oncol Rep 19:1505–1510
Montagna M, Dalla PM, Menin C, Agata S, De Nicolo A, Chieco-Bianchi L, D’Andrea E (2003) Genomic rearrangements account for more than one-third of the BRCA1 mutations in northern Italian breast/ovarian cancer families. Hum Mol Genet 12:1055–1061
Hartmann C, John AL, Klaes R, Hofmann W, Bielen R, Koehler R, Janssen B, Bartram CR, Arnold N, Zschocke J (2004) Large BRCA1 gene deletions are found in 3% of German high-risk breast cancer families. Hum Mutat 24:534
Lindor NM, Lindor RA, Apicella C, Dowty JG, Ashley A, Hunt K, Mincey BA, Wilson M, Smith MC, Hopper JL (2007) Predicting BRCA1 and BRCA2 gene mutation carriers: comparison of LAMBDA, BRCAPRO, Myriad II, and modified Couch models. Fam Cancer 6:473–482
Antoniou AC, Hardy R, Walker L, Evans DG, Shenton A, Eeles R, Shanley S, Pichert G, Izatt L, Rose S, Douglas F, Eccles D, Morrison PJ, Scott J, Zimmern RL, Easton DF, Pharoah PD (2008) Predicting the likelihood of carrying a BRCA1 or BRCA2 mutation: validation of BOADICEA, BRCAPRO, IBIS, Myriad and the Manchester scoring system using data from UK genetics clinics. J Med Genet 45:425–431
van Harssel JJ, van Roozendaal CE, Detisch Y, Brandão RD, Paulussen AD, Zeegers M, Blok MJ, Gómez García EB (2009) Efficiency of BRCAPRO and Myriad II mutation probability thresholds versus cancer history criteria alone for BRCA1/2 mutation detection. Fam Cancer. doi 10.1007/s10689-009-9305-1
Narod SA, Foulkes WD (2004) BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer 4:665–676
Breast Cancer information Core database (BIC). http://resarch.nhgri.nih.gov/BIC. Accessed 26 Nov 2009
Walsh T, Casadei S, Coats KH, Swisher E, Stray SM, Higgins J, Roach KC, Mandell J, Lee MK, Ciernikova S, Foretova L, Soucek P, King MC (2006) Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer. JAMA 295:1379–1388
Casilli F, Tournier I, Sinilnikova OM, Coulet F, Soubrier F, Houdayer C, Hardouin A, Berthet P, Sobol H, Bourdon V, Muller D, Fricker JP, Capoulade-Metay C, Chompret A, Nogues C, Mazoyer S, Chappuis P, Maillet P, Philippe C, Lortholary A, Gesta P, Bezieau S, Toulas C, Gladieff L, Maugard CM, Provencher DM, Dugast C, Delvincourt C, Nguyen TD, Faivre L, Bonadona V, Frebourg T, Lidereau R, Stoppa-Lyonnet D, Tosi M (2006) The contribution of germline rearrangements to the spectrum of BRCA2 mutations. J Med Genet 43:e49
Acknowledgments
We thank Marie Epsteinova for her excellent technical assistance. Special thanks go to Zbynek Halbhuber PhD from KRD Molecular Technologies, who performed experiments with the oligonucleotide-based array CGH technique. This project was supported by the Internal Grant Agency of the Ministry of Health of the Czech Republic, Grant No. NS 10304-3/2009; and the Ministry of Education Youth and Sports Research Project: MSM 0021620808.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ticha, I., Kleibl, Z., Stribrna, J. et al. Screening for genomic rearrangements in BRCA1 and BRCA2 genes in Czech high-risk breast/ovarian cancer patients: high proportion of population specific alterations in BRCA1 gene. Breast Cancer Res Treat 124, 337–347 (2010). https://doi.org/10.1007/s10549-010-0745-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0745-y