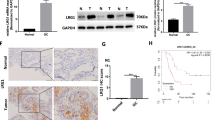
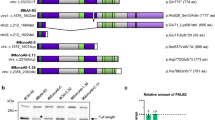
Abstract
Glypican-3 (GPC3) is a proteoglycan involved in proliferation and cell survival. Several reports demonstrated that GPC3 is downregulated in some tumors, such as breast cancer. Previously, we determined that GPC3 reexpression in the murine mammary adenocarcinoma LM3 cells induced an impairment of their invasive and metastatic capacities, associated with a decrease of their motility and an increase of their cell death. We demonstrated that GPC3 inhibits canonical Wnt signaling, as well as it activates non canonical pathway. Now, we identified signaling pathways responsible for the pro-apoptotic role of GPC3 in LM3 cells. We found for the first time that GPC3 inhibits the PI3K/Akt anti-apoptotic pathway while it stimulates the p38MAPK stress-activated one. We report a concomitant modulation of CDK inhibitors as well as of pro- and anti-apoptotic molecules. Our results provide new clues regarding the mechanism involved in the modulation induced by GPC3 of mammary tumor cell growth and survival.









Similar content being viewed by others
Change history
04 September 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10549-023-07120-w
References
Filmus J, Selleck SB (2001) Glypicans: proteoglycans with a surprise. J Clin Invest 108:497–501
Nakato H, Futch TA, Selleck SB (1995) The division abnormally delayed (dally) gene: a putative integral membrane proteoglycan required for cell division pattering during postembryonic development of the nervous system in Drosophila. Development 121:3687–3702
Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N (2001) Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development 126:87–94
Cano-Gauci DF, Song HH, Yang H, McKerlie C, Choo B, Shi W, Pullano R, Piscione TD, Grisaru S, Soon S, Sedlackova L, Tanswell AK, Mak TW, Yeger H, Lockwood GA, Rosenblum ND, Filmus J (1999) Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J Cell Biol 146:255–264
Chiao E, Fisher P, Crisponi L, Deiana M, Dragatsis I, Schlessinger D, Pilia G, Efstratiadis A (2002) Overgrowth of a mouse model of the Simpson-Golabi-Behmel syndrome is independent of IGF signaling. Dev Biol 243:185–206. doi:10.1006/dbio.2001.0554
Garganta CL, Bodurtha JN (1992) Report of another family with Simpson-Golabi-Behmel syndrome and a review of the literature. Am J Med Genet 44:129–135. doi:10.1002/ajmg.1320440202
Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, Cao A, Forabosco A, Schlessinger D (1996) Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet 12:241–247. doi:10.1038/ng0396-241
Dueñas-Gonzalez A, Kaya M, Shi W, Song H, Test JR, Penn LZ, Filmus J (1998) OCI 5/GPC3, a glypican encoded by a gene that is mutated in the Simpson-Golabi-Behmel overgrowth syndrome, induces apoptosis in a cell-specific manner. J Cell Biol 141:1407–1414. doi:10.1083/jcb.141.6.1407
Lin H, Huber R, Schlessinger D, Morin PJ (1999) Frequent silencing of the GPC3 gene in ovarian cancer cell lines. Cancer Res 59:807–810
Murthy SS, Shen T, De Rienzo A, Lee WC, Ferriola PC, Jhanwar SC, Mossman BT, Filmus J, Testa JR (2000) Expression of GPC3, an X-linked recessive overgrowth gene, is silenced in malignant mesothelioma. Oncogene 19:410–416. doi:10.1038/sj.onc.1203322
Xiang YY, Ladeda V, Filmus J (2001) Glypican-3 expression is silenced in human breast cancer. Oncogene 20:7408–7412. doi:10.1038/sj.onc.1204925
Peters MG, Farias E, Colombo L, Filmus J, Puricelli L, Bal de Kier Joffe E (2003) Inhibition of invasion and metastasis by glypican-3 in a syngeneic breast cancer model. Breast Cancer Res Treat 80:221–232. doi:10.1023/A:1024549729256
Weksberg R, Squire JA, Templeton DM (1996) Glypicans: a growing trend. Nat Genet 12:225–227. doi:10.1038/ng0396-225
Song HH, Shi W, Filmus J (1997) OCI-5/rat glypican-3 binds to fibroblast growth factor-2 but not to insulin-like growth factor-2. J Biol Chem 272:7574–7577. doi:10.1074/jbc.272.12.7574
Chiao E, Fisher P, Crisponi L, Deiana M, Dragatsis I, Schlessinger D, Pilia G, Efstratiadis A (2002) Overgrowth of a mouse model of the Simpson-Golabi-Behmel syndrome is independent of IGF signaling. Dev Biol 243:185–206. doi:10.1006/dbio.2001.0554
Capurro MI, Xiang YY, Lobe C, Filmus J (2005) Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res 65:6245–6254. doi:10.1158/0008-5472.CAN-04-4244
Song HH, Shi W, Xiang YY, Filmus J (2005) The loss of glypican-3 induces alterations in Wnt signaling. J Biol Chem 280:2116–2125. doi:10.1074/jbc.M410090200
Stigliano I, Puricelli L, Filmus J, Sogayar MC, Bal de Kier Joffe E, Peters MG (2008) Glypican-3 regulates migration, adhesion and actin cytoskeleton organization in mammary tumor cells through Wnt signaling modulation. Breast Cancer Res Treat 114:251–262. doi:10.1007/s10549-008-0009-2
Urtreger A, Ladeda V, Puricelli L, Rivelli A, Vidal MC, Lustig ES, Bal de Kier Joffé E (1997) Modulation of fibronectin expression and proteolytic activity associated with the invasive and metastatic phenotype in two murine mammary cell lines. Int J Oncol 11:489–496
Bal de Kier Joffé E, Puricelli L, Vidal MC, Lustig ES (1983) Characterization of two murine mammary adenocarcinoma tumors with different metastatic ability. J Exp Clin Cancer Res 2:151–160
Mizushima S, Nagata S (1990) pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res 18:5322. doi:10.1093/nar/18.17.5322
Filmus J, Shi W, Wong ZM, Wong MJ (1995) Identification of a new membrane-bound heparan sulphate proteoglycan. Biochem J 311(Pt 2):561–565
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi:10.1093/nar/29.9.e45
Mitsiades CS, Mitsiades N, Koutsilieris M (2004) The Akt pathway: molecular targets for anti-cancer drug development. Curr Cancer Drug Targets 4:235–256. doi:10.2174/1568009043333032
Gonzalez AD, Kaya M, Shi W, Song H, Testa JR, Penn LZ, Filmus J (1998) OCI-5/GPC3, a glypican encoded by a gene that is mutated in the Simpson-Golabi-Behmel overgrowth syndrome, induces apoptosis in a cell line-specific manner. J Cell Biol 141:1407–1414. doi:10.1083/jcb.141.6.1407
Berger JC, Vander Griend D, Stadler WM, Rinker-Schaeffer C (2004) Metastasis suppressor genes: signal transduction, cross-talk and the potential for modulating the behavior of metastatic cells. Anticancer Drugs 15:559–568. doi:10.1097/01.cad.0000132233.36512.fa
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70. doi:10.1016/S0092-8674(00)81683-9
Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, Cao A, Forabosco A, Schlessinger D (1996) Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet 12:241–247. doi:10.1038/ng0396-241
Nakano K, Vousden KH (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7:683–694. doi:10.1016/S1097-2765(01)00214-3
Vousden KH, Lu X (2002) Live or let die: the cell’s response to p53. Nat Rev Cancer 2:594–604. doi:10.1038/nrc864
Platanias LC (2003) Map kinase signaling pathways and hematologic malignancies. Blood 101:4667–4679. doi:10.1182/blood-2002-12-3647
Baldwin AS Jr (2001) Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest 107:3–6. doi:10.1172/JCI11891
Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A (2005) Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell 20:539–550. doi:10.1016/j.molcel.2005.10.033
Johnson GL, Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911–1912. doi:10.1126/science.1072682
Huang J, Wu L, Tashiro S, Onodera S, Ikejima T (2005) Bcl-2 up-regulation and P-p53 down-regulation account for the low sensitivity of murine L929 fibrosarcoma cells to oridonin-induced apoptosis. Biol Pharm Bull 28:2068–2074. doi:10.1248/bpb.28.2068
She QB, Chen N, Dong Z (2000) ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. J Biol Chem 275:20444–20449. doi:10.1074/jbc.M001020200
Baeg GH, Perrimon N (2000) Functional binding of secreted molecules to heparan sulfate proteoglycans in Drosophila. Curr Opin Cell Biol 12:575–580. doi:10.1016/S0955-0674(00)00134-4
Katoh M (2005) WNT/PCP signaling pathway and human cancer. Oncol Rep 14:1583–1588 (review)
Kuriyama M, Harada N, Kuroda S, Yamamoto T, Nakafuku M, Iwamatsu A, Yamamoto D, Prasad R, Croce C, Canaani E, Kaibuchi K (1996) Identification of AF-6 and canoe as putative targets for Ras. J Biol Chem 271:607–610. doi:10.1074/jbc.271.2.607
Cuadrado A, Lafarga V, Cheung PC, Dolado I, Llanos S, Cohen P, Nebreda AR (2007) A new p38 MAP kinase-regulated transcriptional coactivator that stimulates p53-dependent apoptosis. EMBO J 26:2115–2126. doi:10.1038/sj.emboj.7601657
Acknowledgments
We would like to give our thanks to Guillermo Peluffo for technical assistance and to Dr. Mariana Salatino for her invaluable assistance in responding the reviewer requests. The work was supported by grants from FONCyT (PICT 14088, Préstamo BID 1728/OC-AR; PICT 00220, Préstamo BID 1728/OC-AR) and from the University of Buenos Aires (UBACyT M068).
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Buchanan, L. Puricelli, E. Bal de Kier Joffé, and M. G. Peters are Members of the National Council of Scientific and Technical Research (CONICET).
C. Buchanan and I. Stigliano contributed equally to this paper.
About this article
Cite this article
Buchanan, C., Stigliano, I., Garay-Malpartida, H.M. et al. RETRACTED ARTICLE: Glypican-3 reexpression regulates apoptosis in murine adenocarcinoma mammary cells modulating PI3K/Akt and p38MAPK signaling pathways. Breast Cancer Res Treat 119, 559–574 (2010). https://doi.org/10.1007/s10549-009-0362-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0362-9