Abstract
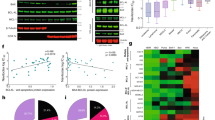
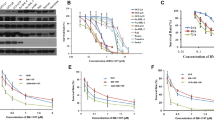
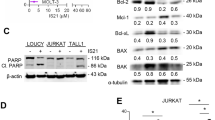
Inhibition or downregulation of Bcl-2 represents a new therapeutic approach to by-pass chemoresistance in cancer cells. Therefore, we explored the potential of this approach in breast cancer cells. Cisplatin and paclitaxel induced apoptosis in a dose-dependent manner in MCF-7 (drug-sensitive) and MDA-MB-231 (drug-insensitive) cells. Furthermore, when we transiently silenced Bcl-2, both cisplatin and paclitaxel induced apoptosis more than parental cells. Dose dependent induction of apoptosis by drugs was enhanced by the pre-treatment of these cells with HA14-1, a Bcl-2 inhibitor. Although the effect of cisplatin was significant on both cell lines, the effect of paclitaxel was much less potent only in MDA-MB-231 cells. To further understand the distinct role of drugs in MDA-MB-231 cells pretreated with HA14-1, caspases and Bcl-2 family proteins were studied. The apoptotic effect of cisplatin with or without HA14-1 pre-treatment is shown to be caspase-dependent. Among pro-apoptotic Bcl-2 proteins, Bax and Puma were found to be up-regulated whereas Bcl-2 and Bcl-xL were down-regulated when cells were pretreated with HA14-1 followed by paclitaxel or cisplatin. Enforced Bcl-2 expression in MDA-MB-231 cells abrogated the sensitizing effect of HA14-1 in cisplatin induced apoptosis. These results suggest that the potentiating effect of HA14-1 is drug and cell type specific and may not only depend on the inhibition of Bcl-2. Importantly, alteration of other pro-apoptotic or anti-apoptotic Bcl-2 family members may dictate the apoptotic response when HA14-1 is combined with chemotherapeutic drugs.






Similar content being viewed by others
References
Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC (2005) Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17(3):393–403. doi:10.1016/j.molcel.2004.12.030
Reed JC (2003) Apoptosis targeted therapies for cancer. Cancer Cell 3:17–22. doi:10.1016/S1535-6108(02)00241-6
Letai A (2005) Pharmacological manipulation of Bcl-2 family members to control cell death. J Clin Invest 115:2648–2655. doi:10.1172/JCI26250
Wang JL, Zhang ZJ, Choksi S, Shan S, Lu Z, Croce CM et al (2000) Cell permeable Bcl-2 binding peptides: a chemical approach to apoptosis induction in tumor cells. Cancer Res 60:1498–1502
Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM et al (2000) Structure based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA 97:7124–7129. doi:10.1073/pnas.97.13.7124
Manero F, Gaultier F, Gallenne T, Cauquil N, Gree D, Cartron PF et al (2006) The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res 66:2757–2764. doi:10.1158/0008-5472.CAN-05-2097
Zimmermann AK, Loucks FA, Le SS, Butts BD, Florez-McClure ML, Bouchard RJ et al (2005) Distinct mechanisms of neuronal apoptosis are triggered by antagonism of Bcl-2/Bcl-x(L) versus induction of the BH3-only protein Bim. J Neurochem 94:22–36. doi:10.1111/j.1471-4159.2005.03156.x
Chen J, Freeman A, Liu J, Dai Q, Lee RM (2002) The apoptotic effect of HA14-1, a Bcl-2 interacting small molecular compound, requires Bax translocation and is enhanced by PK11195. Mol Cancer Ther 1:961–967
Tian D, Das SG, Doshi JM, Peng J, Lin J, Xing C (2008) sHA14-1, is a stable and ROS free antagonist against anti-apoptotic Bcl-2 proteins, bypasses drug resistances and synergizes cancer therapies in human leukemia cell. Cancer Lett 259:198–208. doi:10.1016/j.canlet.2007.10.012
Enyedy IJ, Ling Y, Nacro K, Tomita Y, Wu X, Cao Y (2001) Discovery of small-molecule inhibitors of Bcl-2 through structure based computer screening. J Med Chem 44:4313–4324. doi:10.1021/jm010016f
An J, Chervin AS, Nie A, Ducoff HS, Huang Z (2006) Overcoming the radioresistance of prostate cells with a novel Bcl-2 inhibitor. Oncogene 26(5):652–661. doi:10.1038/sj.onc.1209830
Oliver L, Mahe B, Gree R, Vallette FM, Juin P (2007) HA14-1, a small molecule inhibitor of Bcl-2 bypasses chemoresistance in leukemia cells. Leuk Res 31(6):859–863. doi:10.1016/j.leukres.2006.11.010
Pei X, Dai Y, Grant S (2003) The proteasome inhibitor bortezomib promotes mitochondrial injury and apoptosis induced by the small molecule Bcl-2 inhibitor, HA14-1 in multiple myeloma cells. Leukemia 17:2036–2045. doi:10.1038/sj.leu.2403109
Sinicrope FA, Penington RC, Tang XM (2004) Tumor necrosis factor related apoptosis inducing ligand induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA14-1 in human colon cancer cells. Clin Cancer Res 10:8284–8292. doi:10.1158/1078-0432.CCR-04-1289
Su Y, Zhang X, Sinko PJ (2007) Exploitation of drug induced Bcl-2 overexpression for restoring normal apoptosis function: a promising new approach to the treatment of multidrug resistant cancer. Cancer Lett 253:115–123. doi:10.1016/j.canlet.2007.01.018
Moon D, Kim M, Choi YH, Kim ND, Chang JH, Kim G (2008) Bcl-2 overexpression attenuates SP600125 induced apoptosis in human leukemia U937 cells. Cancer Lett 264:316–325. doi:10.1016/j.canlet.2008.02.011
Lickliter JD, Wood NJ, Johnson L, McHugh G, Tan J, Wood F et al (2003) HA14-1 selectively induces apoptosis in Bcl-2 overexpressing leukemia/lymphoma cells, and enhances cytarabine induced cell death. Leukemia 17:2074–2080. doi:10.1038/sj.leu.2403102
Pei XY, Dai Y, Grant S (2004) The small-molecule Bcl-2 inhibitor HA14-1 interacts synergistically with flavopiridol to induce mitochondrial injury and apoptosis in human myeloma cells through a free radical dependent and Jun NH2 terminal kinase dependent mechanism. Mol Cancer Ther 3:1513–1524
Milella M, Estrov Z, Kornblau SM, Carter BZ, Konopleva M, Tari A et al (2002) Synergistic induction of apoptosis by simultaneous disruption of the Bcl-2 and MEK/MAPK pathways in acute myelogenous leukemia. Blood 99:3461–3464. doi:10.1182/blood.V99.9.3461
Okani JI, Rustgi AK (2001) Paclitaxel induces prolonged activation of the Ras/MEK/ERK pathway independently of activation of the programmed cell death machinery. J Biol Chem 276:19555–19564. doi:10.1074/jbc.M011164200
Ling Y, Zhong Y, Perez-Soler R (2001) Disruption of cell adhesion and caspase-mediated proteolysis of beta- and gamma-catenins and APC protein in paclitaxel-induced apoptosis. Mol Pharmacol 59:593–603
Skommer J, Wlodkowic D, Matto M, Eray M, Pelkonen J (2006) HA14-1, a small molecule Bcl-2 antagonist, induces apoptosis and modulates action of selected anticancer drugs in follicular B lymphoma B cells. Leuk Res 30:322–331. doi:10.1016/j.leukres.2005.08.022
Srivastava RK, Mi QS, Hardwick JM, Longo DL (1999) Deletion of the loop region of Bcl-2 completely blocks paclitaxel-induced apoptosis. Proc Natl Acad Sci USA 96(7):3775–3780. doi:10.1073/pnas.96.7.3775
Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B (2001) Puma induces the rapid apoptosis of colorectal cancer cells. Mol Cell 7(3):673–682. doi:10.1016/S1097-2765(01)00213-1
Wang X, Li M, Wang J, Yeung CM, Zhang H, Kung HF, Jiang B, Lin MC (2006) The BH3-only protein, PUMA, is involved in oxaliplatin-induced apoptosis in colon cancer cells. Biochem Pharmacol 71(11):1540–1550. doi:10.1016/j.bcp.2006.02.011
Ming L, Wang P, Bank A, Yu J, Zhang L (2006) Puma dissociates Bax and BCL-XL to induce apoptosis in colon cancer cells. J Biol Chem 281:16034–16042. doi:10.1074/jbc.M513587200
Liu Z, Lu H, Shi H, Du Y, Yu J, Gu S, Chen X, Liu KJ, Hu CA (2005) Puma overexpression induces reactive oxygen species generation and proteasome-mediated stathmin degradation in colorectal cancer cells. Cancer Res 65(5):1647–1654. doi:10.1158/0008-5472.CAN-04-1754
Lowe SW, Ruley HE, Jacks T, Housman DE (1993) p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74:957–967. doi:10.1016/0092-8674(93)90719-7
Stewart DJ (2007) Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol 63(1):12–31. doi:10.1016/j.critrevonc.2007.02.001
Acknowledgment
This work was partially supported by Turkish Association for Cancer Research and Control, Terry Fox Cancer Research Grant-35-D/882.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2009_343_MOESM1_ESM.pdf
Quantitative mRNA levels were determined by quantitative real time PCR assay following transfection of MCF-7 and MDA-MB-231 cells with Bcl-2 siRNA and scramble siRNA for 48 h. Bcl-2 mRNA copy number was calculated using standard curve of β-actin. These values are normalized by dividing with β-actin. Columns represent mean (±SEM) of independent two experiments and each experiment was repeated three times (DOC 22 kb)
Rights and permissions
About this article
Cite this article
Arisan, E.D., Kutuk, O., Tezil, T. et al. Small inhibitor of Bcl-2, HA14-1, selectively enhanced the apoptotic effect of cisplatin by modulating Bcl-2 family members in MDA-MB-231 breast cancer cells. Breast Cancer Res Treat 119, 271–281 (2010). https://doi.org/10.1007/s10549-009-0343-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0343-z