Abstract
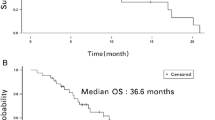
The combination therapy of doxorubicin and trastuzumab has been proven to be highly effective for metastatic breast cancer (MBC) patients with Her2/neu over-expressing tumors. However, this regimen is characterized by frequent cardiac toxicity, occurring in 27% of all treated patients and aggravating when the two substances are given concurrently. Pegylated liposomal doxorubicin (PLD) as a single agent reduces significantly cardiac toxicity and maintains efficacy compared to conventional doxorubicin. This prospective open labeled, multicenter phase II study assessed the potential cardiotoxicity and efficacy of PLD and trastuzumab as first and second line combination therapy in Her2/neu over-expressing MBC patients. Patients with Her2 over-expressing, measurable MBC with a baseline left ventricular ejection fraction (LVEF) ≥50% were treated with PLD 40 mg/m2 every 4 weeks for 6 up to 9 cycles and weekly trastuzumab (4 mg/kg loading dose, then 2 mg/kg). Cardiotoxicity was defined as the appearance of clinical signs or symptoms of congestive heart failure in combination with a decrease in LVEF ≤44% or ≥10 units below the normal value of 50% in the obligatory, subsequently performed transthoracic echocardiography. Due to conflicting interests, the planned accrual goal of 30 patients was not reached. Finally 16 patients were enrolled. Ten patients presented with more than one metastatic site and six of them were in second-line therapy. The median LVEF in the study cohort was 66.1 ± 8.68% at baseline, 62.7 ± 5.11% after 6 cycles of therapy, 64.4 ± 7.61% at the first follow up and did not change significantly (61.0 ± 5.56% even at the 5th follow-up). Six out of 12 assessable patients (50.0%) demonstrated a clinical benefit and after a median follow-up of 15.4 months a median progression free survival of 9.67 and a median overall survival of 16.23 months. Non-cardiac side effects were mild with only 3 CTC grade 3 events of 247 treatment cycles (1.2%) and no grade 4 toxicities. The combination of PLD and trastuzumab in patients with Her2/neu over-expressing metastatic breast cancer is a safe, feasible and effective therapy. However, cardiac function should be monitored at close intervals. Due to the promising clinical response rates and mild toxicity profile in this prognostically unfavorable group, this combination therapy should be evaluated in larger studies.



Similar content being viewed by others
References
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A et al (1989) Studies of the Her-2/Neu proto-oncogene in human breast and ovarian cancer. Science 244(4905):707–712. doi:10.1126/science.2470152
Ravdin PM, Chamness GC (1995) The C-Erbb-2 Proto-Oncogene as a prognostic and predictive marker in breast cancer: a paradigm for the development of other macromolecular markers—a review. Gene 159(1):19–27. doi:10.1016/0378-1119(94)00866-Q
Lohrisch C, Piccart M (2001) An overview of Her2. Semin Oncol 28(6 (suppl 18)):3–11. doi:10.1053/sonc.2001.29713
Norton L, Slamon D, Leyland-Jones B et al (1999) Overall survival (OS) advantage to simultaneous chemotherapy (CRx) plus the humanized anti-HER2 monoclonal antibody Herceptin (H) in HER2-overexpressing (HER2 +) metastatic breast cancer (MBC). Proc Am Soc Clin Oncol 18:127a
Vogel CL, Franco SX (2003) Clinical experience with trastuzumab (Herceptin). Breast J 9(6):452–462. doi:10.1046/j.1524-4741.2003.09602.x
O’Shaughnessy JA (2003) Pegylated liposomal doxorubicin in the treatment of breast cancer. Clin Breast Cancer 4(5):318–328. doi:10.3816/CBC.2003.n.037
Pegram MD, Finn RS, Arzoo K, Beryt M, Pietras RJ, Slamon DJ (1997) The effect of Her-2/Neu overexpression on chemotherapeutic drug sensitivity in human breast and ovarian cancer cells. Oncogene 15(5):537–547. doi:10.1038/sj.onc.1201222
Pegram M, Hsu S, Lewis G, Pietras R, Beryt M, Sliwkowski M, Coombs D, Baly D, Kabbinavar F, Slamon D (1999) Inhibitory effects of combinations of Her-2/Neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 18(13):2241–2251. doi:10.1038/sj.onc.1202526
Slamon D, Pegram M (2001) Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin Oncol 28(Suppl 3):13–19. doi:10.1053/sonc.2001.22812
Gianni L, Salvatorelli E, Minotti G (2007) Anthracycline cardiotoxicity in breast cancer patients: synergism with trastuzumab and taxanes. Cardiovasc Toxicol 7(2):67–71. doi:10.1007/s12012-007-0013-5
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792. doi:10.1056/NEJM200103153441101
Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, Hagerty KL, Somerfield MR, Vaughn DJ (2007) American Society of Clinical Oncology Clinical Evidence on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 25:3991–4008. doi:10.1200/JCO.2007.10.9777
Chia S, Clemons M, Martin LA, Rodgers A, Gelmon K, Pond GR, Panasci L (2006) Pegylated liposomal doxorubicin and trastuzumab in her-2 overexpressing metastatic breast cancer: a multicenter phase II trial. J Clin Oncol 24(18):2773–2778. doi:10.1200/JCO.2005.03.8331
Andreopoulou E, Gaiotti D, Kim E, Volm M, Oratz R, Freedberg R, Downey A, Vogel CL, Chia S, Muggia F (2007) Feasibility and cardiac safety of pegylated liposomal doxorubicin plus trastuzumab in heavily pretreated patients with recurrent HER2-overexpressing metastatic breast cancer. Clin Breast Cancer 7(9):690–696. doi:10.3816/CBC.2007.n.028
Theodoulou M, Campos SM, Batist G et al (2002) TLC D99 (D, Myocet) and herceptin (H) is safe in advanced breast cancer (ABC): final cardiac safety and efficacy analysis. Proc Am Soc Clin Oncol 21:55a (Abstr 216)
Trigo J, Climent MA, Lluch A et al. (2003) Liposomal doxorubicin (Myocet) in combination with Herceptin and paclitaxel is active and well tolerated in patients with HER2-positive locally advanced or metastatic breast cancer (LA/MBC): a phase II study. Poster presented at the 26th annual san antonio breast cancer symposium, San Antonio, Texas, 3–6 December 2003
Telli ML, Hunt SA, Carlson RW, Guardino AE (2007) Trastuzumab related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol 25:3525–3533. doi:10.1200/JCO.2007.11.0106
Viani GA, Afonso SL, Stefano EJ, De Fendi LI, Soares FV (2007) Adjuvant trastuzumab in the treatment of her2 positive early breast cancer: a metaanalysis of published randomized trials. BMC Cancer 7:153–164. doi:10.1186/1471-2407-7-153
Gianni L, Semiglazov V, Manikhas GM et al. (2007) Neoadjuvant trastuzumab plus doxorubicin, paclitaxel and CMF in locally advanced breast cancer (NOAH trial): feasibility, safety and antitumor effects. ASCO Breast Meeting (2007) (Abstr 144)
Press MF, Cordon-Cardo C, Slamon DJ (1990) Expression of the Her-2/Neu proto-oncogene in normal human adult and fetal tissues. Oncogene 5(7):953–962
Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL (2006) Chemotherapy and cardiotoxicity in older breast cancer patients: a population based study. J Clin Oncol 34:8597–8605
Pai VB, Nahata MC (2000) Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf 22(4):263–302. doi:10.2165/00002018-200022040-00002
Ewer MS, Lippmann SM (2005) Typ II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol 23:2900–2902. doi:10.1200/JCO.2005.05.827
Ewer MS, Martin FJ, Henderson C, Shapiro CL, Benjamin RS, Gabizon AA (2004) Cardiac safety of liposomal anthracyclines. Semin Oncol 31(Suppl 13):161–181. doi:10.1053/j.seminoncol.2004.08.006
Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, Welles L, Winer E (2002) Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 94(1):25–36. doi:10.1002/cncr.10201
Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, Henderson R, Berry G, Gabizon A (2000) Pegylated liposomal doxorubicin (Doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol 11(8):1029–1033
Theodoulou M, Hudis C (2004) Cardiac profiles of liposomal anthracyclines: greater cardiac safety versus conventional doxorubicin? Cancer 100(10):2052–2063
O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C, CAELYX Breast Cancer Study Group (2004) Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 15(3):440–449. doi:10.1093/annonc/mdh097
Perez EA, Rodeheffer R (2004) Clinical cardiac tolerability of trastuzumab. J Clin Oncol 22(2):322–329. doi:10.1200/JCO.2004.01.120
Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D (2002) Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20(5):1215–1221. doi:10.1200/JCO.20.5.1215
Wolff AC, Bonetti M, Sparano JA, Wang M, Davidson NE, on behalf of ECOG (2003) Cardiac safety of trastuzumab (H) in combination with pegylated liposomal doxorubicin (D) and docetaxel (T) in HER2-positive metastatic breast cancer (MBC): preliminary results of the eastern cooperative oncology group trial E3198. Proc Am Soc Clin Oncol 22:18a (abstr 70)
Untch M, Tjulandin S, Jonat W, Meerpohl H, Lichinitser M, Manikhas GM, Jänicke F, Muscholl M, pauschinger M, Thomssen C, Lehle M (2007) “Evaluation of first line trastuzumab in combination with epirubicin/cyclophosphamidefor patients with Her2-positive metastatic breast cancer” SABCS 2007 (abstract 4058)
Von Minckwitz G, Rezai M, Loibl S, Fasching P, Huober J, Tesch H, Bauerfeind I, Hilfrich J, Mehta K, Untch M (2007) “GeparQuattro: first interim safety analysis of a phase III trial exploring the efficacy of capecitabine and trastuzumab given concomitantly or in sequence to EC-Doc as neoadjuvant treatment of primary breast cancer”. ASCO 2007 (abstract 222)
Acknowledgments
The authors would like thank Jörg Lebauer for statistical data analysis and the companies Essex Pharma and Hoffmann-La Roche for their support of the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Elmar Stickeler and Maximilian Klar contributed equally to this work.
Rights and permissions
About this article
Cite this article
Stickeler, E., Klar, M., Watermann, D. et al. Pegylated liposomal doxorubicin and trastuzumab as 1st and 2nd line therapy in her2/neu positive metastatic breast cancer: a multicenter phase II trial. Breast Cancer Res Treat 117, 591–598 (2009). https://doi.org/10.1007/s10549-008-0306-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-0306-9