Abstract
Promoter-CpG island hypermethylation has been proposed as an alternative mechanism to inactivate BRCA1 in the breast where somatic mutations of BRCA1 are rare. To better understand breast cancer etiology and progression, we explored the association between BRCA1 promoter methylation status and prognostic factors as well as survival among women with breast cancer. Promoter methylation of BRCA1 was assessed in 851 archived tumor tissues collected from a population-based study of women diagnosed with invasive or in situ breast cancer in 1996–1997, and who were followed for vital status through the end of 2002. About 59% of the tumors were methylated at the promoter of BRCA1. The BRCA1 promoter methylation was more frequent in invasive cancers (P = 0.02) and among premenopausal cases (P = 0.05). BRCA1 promoter methylation was associated with increased risk of breast cancer-specific mortality (age-adjusted HR 1.71; 95% CI: 1.05–2.78) and all-cause mortality (age-adjusted HR 1.49; 95% CI: 1.02–2.18). Neither dietary methyl intakes in the year prior to the baseline interview nor the functional polymorphisms in one-carbon metabolism were associated with BRCA1 methylation status. Our study is the first epidemiological investigation on the prognostic value of BRCA1 promoter methylation in a large population-based cohort of breast cancer patients. Our results indicate that BRCA1 promoter methylation is an important factor to consider in predicting breast cancer survival.


Similar content being viewed by others
References
Landis SH, Murray T, Bolden S et al. (1999) Cancer statistics, 1999. CA Cancer J Clin 49:8–31
Russo J, Yang X, Hu YF et al (1998) Biological and molecular basis of human breast cancer. Front Biosci 3:D944–D960
Baylin SB, Herman JG, Graff JR et al (1998) Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res 72:141–196. doi:10.1016/S0065-230X(08)60702-2
Ehrlich M (2002) DNA methylation in cancer: too much, but also too little. Oncogene 21:5400–5413. doi:10.1038/sj.onc.1205651
Herman JG (1999) Hypermethylation of tumor suppressor genes in cancer. Semin Cancer Biol 9:359–367. doi:10.1006/scbi.1999.0138
Widschwendter M, Jones PA (2002) DNA methylation and breast carcinogenesis. Oncogene 21:5462–5482. doi:10.1038/sj.onc.1205606
Ralhan R, Kaur J, Kreienberg R et al (2007) Links between DNA double strand break repair and breast cancer: accumulating evidence from both familial and nonfamilial cases. Cancer Lett 248:1–17. doi:10.1016/j.canlet.2006.06.004
Miki Y, Swensen J, Shattuck-Eidens D et al (1994) A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266:66–71. doi:10.1126/science.7545954
Hopper JL (2001) Genetic epidemiology of female breast cancer. Semin Cancer Biol 11:367–374. doi:10.1006/scbi.2001.0392
Yang X, Yan L, Davidson NE (2001) DNA methylation in breast cancer. Endocr Relat Cancer 8:115–127. doi:10.1677/erc.0.0080115
Birgisdottir V, Stefansson OA, Bodvarsdottir SK et al (2006) Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res 8:R38. doi:10.1186/bcr1522
Butcher DT, Rodenhiser DI (2007) Epigenetic inactivation of BRCA1 is associated with aberrant expression of CTCF and DNA methyltransferase (DNMT3B) in some sporadic breast tumours. Eur J Cancer 43:210–219. doi:10.1016/j.ejca.2006.09.002
Pathology of familial breast cancer: differences between breast cancers in carriers of BRCA1 or BRCA2 mutations and sporadic cases (1997) Breast Cancer Linkage Consortium. Lancet 349:1505–1510. doi:10.1016/S0140-6736(96)10109-4
Robson ME, Chappuis PO, Satagopan J et al (2004) A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res 6:R8–R17. doi:10.1186/bcr658
Stoppa-Lyonnet D, Ansquer Y, Dreyfus H et al (2000) Familial invasive breast cancers: worse outcome related to BRCA1 mutations. J Clin Oncol 18:4053–4059
Chappuis PO, Kapusta L, Begin LR et al (2000) Germline BRCA1/2 mutations and p27(Kip1) protein levels independently predict outcome after breast cancer. J Clin Oncol 18:4045–4052
Goffin JR, Chappuis PO, Begin LR et al (2003) Impact of germline BRCA1 mutations and overexpression of p53 on prognosis and response to treatment following breast carcinoma: 10-year follow up data. Cancer 97:527–536. doi:10.1002/cncr.11080
Moller P, Evans DG, Reis MM et al (2007) Surveillance for familial breast cancer: Differences in outcome according to BRCA mutation status. Int J Cancer 121:1017–1020. doi:10.1002/ijc.22789
Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, panthothenic acid, biotin, and choline (1998) National Academies Press, Washington, DC
Christman JK, Sheikhnejad G, Dizik M et al (1993) Reversibility of changes in nucleic acid methylation and gene expression induced in rat liver by severe dietary methyl deficiency. Carcinogenesis 14:551–557. doi:10.1093/carcin/14.4.551
Fowler BM, Giuliano AR, Piyathilake C et al (1998) Hypomethylation in cervical tissue: is there a correlation with folate status? Cancer Epidemiol Biomarkers Prev 7:901–906
Rampersaud GC, Kauwell GP, Hutson AD et al (2000) Genomic DNA methylation decreases in response to moderate folate depletion in elderly women. Am J Clin Nutr 72:998–1003
Jacob RA, Gretz DM, Taylor PC et al (1998) Moderate folate depletion increases plasma homocysteine and decreases lymphocyte DNA methylation in postmenopausal women. J Nutr 128:1204–1212
Paz MF, Avila S, Fraga MF et al (2002) Germ-line variants in methyl-group metabolism genes and susceptibility to DNA methylation in normal tissues and human primary tumors. Cancer Res 62:4519–4524
Stern LL, Mason JB, Selhub J et al (2000) Genomic DNA hypomethylation, a characteristic of most cancers, is present in peripheral leukocytes of individuals who are homozygous for the C677T polymorphism in the methylenetetrahydrofolate reductase gene. Cancer Epidemiol Biomarkers Prev 9:849–853
Friso S, Choi SW, Girelli D et al (2002) A common mutation in the 5, 10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci USA 99:5606–5611. doi:10.1073/pnas.062066299
Chen J, Gammon MD, Chan W et al (2005) One-carbon metabolism, MTHFR polymorphisms, and risk of breast cancer. Cancer Res 65:1606–1614. doi:10.1158/0008-5472.CAN-04-2630
Xu X, Gammon MD, Zhang H et al (2007) Polymorphisms of one-carbon metabolizing genes and risk of breast cancer in a population-based study. Carcinogenesis 28:1504–1509
Xu X, Gammon MD, Zeisel SH et al (2008) Choline metabolism and risk of breast cancer in a population-based study. FASEB J [Epub ahead of print]
Gammon MD, Neugut AI, Santella RM et al (2002) The Long Island breast cancer study project: description of a multi-institutional collaboration to identify environmental risk factors for breast cancer. Breast Cancer Res Treat 74:235–254. doi:10.1023/A:1016387020854
Cleveland RJ, Eng SM, Abrahamson PE et al (2007) Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol Biomarkers Prev 16:1803–1811. doi:10.1158/1055-9965.EPI-06-0889
Gammon MD, Santella RM, Neugut AI et al (2002) Environmental toxins and breast cancer on Long Island. I. Polycyclic aromatic hydrocarbon DNA adducts. Cancer Epidemiol Biomarkers Prev 11:677–685
Gaudet MM, Britton JA, Kabat GC et al (2004) Fruits, vegetables, and micronutrients in relation to breast cancer modified by menopause and hormone receptor status. Cancer Epidemiol Biomarkers Prev 13:1485–1494
McShane LM, Altman DG, Sauerbrei W et al (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235. doi:10.1007/s10549-006-9242-8
Zeisel SH, Mar MH, Howe JC et al (2003) Concentrations of choline-containing compounds and betaine in common foods. J Nutr 133:1302–1307
Hosmer DW (1999) Applied survival analysis : regression modeling of time to event data. Wiley, New York
Rosen EM, Fan S, Pestell RG et al (2003) BRCA1 gene in breast cancer. J Cell Physiol 196:19–41. doi:10.1002/jcp. 10257
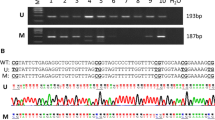
Herman JG, Graff JR, Myohanen S et al (1996) Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 93:9821–9826. doi:10.1073/pnas.93.18.9821
Hilakivi-Clarke L (2000) Estrogens, BRCA1, and breast cancer. Cancer Res 60:4993–5001
Fan S, Wang J, Yuan R et al (1999) BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science 284:1354–1356. doi:10.1126/science.284.5418.1354
Fan S, Ma YX, Wang C et al (2001) Role of direct interaction in BRCA1 inhibition of estrogen receptor activity. Oncogene 20:77–87. doi:10.1038/sj.onc.1204073
Liebens FP, Carly B, Pastijn A et al (2007) Management of BRCA1/2 associated breast cancer: a systematic qualitative review of the state of knowledge in 2006. Eur J Cancer 43:238–257. doi:10.1016/j.ejca.2006.07.019
Yoshikawa K, Honda K, Inamoto T et al (1999) Reduction of BRCA1 protein expression in Japanese sporadic breast carcinomas and its frequent loss in BRCA1-associated cases. Clin Cancer Res 5:1249–1261
Perez-Valles A, Martorell-Cebollada M, Nogueira-Vazquez E et al (2001) The usefulness of antibodies to the BRCA1 protein in detecting the mutated BRCA1 gene. An immunohistochemical study. J Clin Pathol 54:476–480. doi:10.1136/jcp. 54.6.476
Al-Mulla F, Abdulrahman M, Varadharaj G et al (2005) BRCA1 gene expression in breast cancer: a correlative study between real-time RT-PCR and immunohistochemistry. J Histochem Cytochem 53:621–629. doi:10.1369/jhc.4A6544.2005
Wilson CA, Ramos L, Villasenor MR et al (1999) Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet 21:236–240. doi:10.1038/6029
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the National Institutes of Health (CA109753 to JC; DK55865 to SZ) and in part by grants from Department of Defense (BC031746), National Cancer Institute and the National Institutes of Environmental Health and Sciences (UO1CA/ES66572, UO1CA66572, P30CA013696, P30ES09089 and P30ES10126); and by the University of North Carolina Clinical Nutrition Research Unit (DK56350) and Center for Environmental Health and Susceptibility (ES10126). Xu, X. is a recipient of the Predoctoral Traineeship Award (W81XWH-06-1-0298) of Department of Defense Breast Cancer Research Program.
Rights and permissions
About this article
Cite this article
Xu, X., Gammon, M.D., Zhang, Y. et al. BRCA1 promoter methylation is associated with increased mortality among women with breast cancer. Breast Cancer Res Treat 115, 397–404 (2009). https://doi.org/10.1007/s10549-008-0075-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-0075-5