Abstract
Purpose
We have previously demonstrated in a pilot study of 348 invasive breast cancers that mast cell (MC) infiltrates within primary breast cancers are associated with a good prognosis. Our aim was to verify this finding in a larger cohort of invasive breast cancer patients and examine the relationship between the presence of MCs and other clinical and pathological features.
Experimental design
Clinically annotated tissue microarrays (TMAs) containing 4,444 cases were constructed and stained with c-Kit (CD-117) using standard immunoperoxidase techniques to identify and quantify MCs. For statistical analysis, we applied a split-sample validation technique. Breast cancer specific survival was analyzed by Kaplan–Meier [KM] method and log rank test was used to compare survival curves.
Results
Survival analysis by KM method showed that the presence of stromal MCs was a favourable prognostic factor in the training set (P = 0.001), and the validation set group (P = 0.006). X-tile plot generated to define the optimal number of MCs showed that the presence of any number of stromal MCs predicted good prognosis. Multivariate analysis showed that the MC effect in the training set (Hazard ratio [HR] = 0.804, 95% Confidence interval [CI], 0.653–0.991, P = 0.041) and validation set analysis (HR = 0.846, 95% CI, 0.683–1.049, P = 0.128) was independent of age, tumor grade, tumor size, lymph node, ER and Her2 status.
Conclusions
This study concludes that stromal MC infiltration in invasive breast cancer is an independent good prognostic marker and reiterates the critical role of local inflammatory responses in breast cancer progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mast cells (MCs) are part of the innate immune system and are recruited to and activated in the microenvironment of a developing tumor. MCs originate from multipotential hemopoietic stem cells in the bone marrow, and express the proto-oncogene c-kit, a transmembrane type III tyrosine kinase receptor protein [1]. After leaving the vascular bed they differentiate and acquire functional maturity.
MC infiltrates have been described in a variety of human cancers, including non-small-cell lung cancer [2–4], breast cancer [5], colorectal cancer [6], basal cell carcinoma [7] and pulmonary adenocarcinoma [8]. MCs are attracted to the tumor by tumor-derived chemo attractants where they either degranulate to release potential tumor cytotoxic compounds or become innocent bystanders depending on local tumor conditions [9]. There is controversy about the pro- and anti-tumorigenic effects of MCs in different cancers. The accumulation of MCs has been associated with enhanced growth and invasion of several human cancers [10]. On the other hand, MC infiltration has been associated with good prognosis in breast [11, 12] ovarian [13], lung [14] and colorectal [15] carcinomas.
Murine models have shown that tumor incidence and growth are inversely correlated with the MC density in MC-deficient mice compared to normal [16].
Our previously published pilot report of 348 case series of breast carcinomas showed that the presence of stromal MCs correlated with a good prognosis (P = 0.0036) in invasive breast cancer [11].We present here a large tissue microarray (TMA) study of 4,444 cases of invasive breast carcinomas with clinical outcome data. This study validates the independent prognostic significance of stromal MCs in breast cancer.
Materials and methods
Patient selection
A total of 4,620 archival samples from patients with invasive breast carcinoma referred to the British Columbia Cancer Agency between January 1986 and September 1992 were used for TMA construction. The Clinical Research Ethics Board of the University of British Columbia approved the study. Patients with in-situ disease, metastatic disease at presentation, and male breast cancer were excluded from analysis, thus bringing the final tally to 4,444 cases. This represents 34% of all patients diagnosed with breast cancer in the province of British Columbia during this time period. This large, well characterized cohort is derived from a consecutive series of patients who were referred to the BC Cancer Agency for consultation and had tumor samples sent to a central laboratory at the Vancouver General Hospital for estrogen receptor (ER) status. Consequently, for all of these patients we have available detailed demographic and outcome data, as well as formalin fixed paraffin embedded (FFPE) primary tumor samples for immunohistochemical analysis. Available clinical information includes age, histology, tumor grade, tumor size, lymph node status, type of local and adjuvant systemic therapy, and dates of first recurrence and death. Clinical and pathological variables were determined following well-established criteria. A portion of this cohort of patients was recently used in a population study validating the on-line breast cancer prognostic calculator ADJUVANT! Online [17].
TMA construction
The Vancouver Hospital ER laboratory retained single archival tumor blocks from each case in this patient cohort. The material had been frozen prior to neutral buffered formalin fixation. All the paraffin sections were first stained with H&E and reviewed by a pathologist. Representative tumor areas were circled and matched with the donor blocks. 0.6-mm cores were punched out from the donor blocks and embedded 1 mm apart in 17 recipient blocks using Tissue Microarrayer (Beecher instruments Silver Springs, MD). These tissue arrays have been used to test the new ER SP1 antibody that improves the sensitivity for detecting ER by IHC [18].
Immunohistochemistry (IHC)
Sections from TMA were cut at 4 μm and immunostained with antibody to KIT (CD117), a transmembrane tyrosine kinase acting as a type III receptor for MC growth factor. Slides were also concurrently stained for ER and Her2 using standard immunoperoxidase techniques. The antibodies and antigen retrieval methods are summarized in Table 1.
Immunohistochemical image processing and scoring
The stained slides were digitally scanned with a BLISS automated digital imaging microscope (Bacus Laboratories, Lombard, IL) which consists of a microscope with a scanning stage, video camera and software designed for scanning TMAs. A relational database was constructed using identification information and immunohistochemistry scores for each tissue core in the microarrays. An internet website was then constructed using this database and a WebSlideViewer Java applet provided by the manufacturer to view the microarray images and allow for an image zooming functionality. This website is publicly accessible through http://www.gpecimage.ubc.ca/tma/web/viewer.php.
The slides were scored manually by two independent pathologists, blinded to the clinical outcome, as previously described [11]. Total number of stained MCs in each core was recorded. ER status was assessed using the rabbit monoclonal antibody (SP1) antibody [18]. The fractions of ER positive tumor nuclei were scored as 0 (<1%), 1 (1–25%), 2 (25–75%), and 3 (>75%). Her2 SP3 rabbit monoclonal from LabVision (NeoMarkers) was used to stain the TMA slides and were scored using Hercept test® (Dako Corporation, Carpinteria, CA) scoring system. Final Her2 score was derived using both IHC and Fluorescent in situ hybridization (FISH) assays. Cases with Her2 IHC Herceptest score = 3, were scored as positive. Those cases with Her2 IHC Herceptest score = 2 were re-evaluated using FISH assay, and only those cases with Her2 FISH amplification ratio ≥2.0 were scored as Her2 positive. C-Kit and ER scores were binarized for statistical analysis as follows: CD-117: 0 = no MCs; 1 = any MCs; ER: 0 ≤ 1% nuclei stained; 1 ≥ 1% nuclei stained. We excluded cases for which it was not possible to assign a score to the immunostaining (insufficient invasive tumor in the core, or missing core).
Statistical analysis
The raw scores were entered into an Excel database and each TMA core was assigned a unique core ID number. The spreadsheet was then processed utilizing TMA-Deconvoluter 1.06 software that had been adapted for TMA analysis [19]. A database was created by incorporation of all deconvoluted marker data into the clinicopathologic patient database.
We applied a split-sample validation technique for our statistical analysis. Our 4,444 patient cohort was stratified into eight subgroups (Table 2) based on adjuvant treatment received and then randomized into equal sized training and validation sets. The two groups were balanced with respect to treatment received; but there are also no significant differences in clinical or pathological variables, including age, tumor size and grade, nodal status, and ER status. For this study, and future studies using this TMA, the primary investigator is given access to all clinical, outcome, and TMA data from the training set only. The training set is used to generate and refine hypotheses regarding the biomarker under study. Significant findings are then formally presented at a bimonthly joint scientific group meeting of the Genetic Pathology Evaluation Centre (GPEC, a collaborative group of scientists and pathologists) and Breast Cancer Outcomes Unit (BCOU, a group of oncologists and epidemiologists based at the BC Cancer Agency). Those findings considered to be of clinical and scientific interest are then re-tested on the validation set. A separate researcher who did not participate in the training set analysis performs the re-testing on the validation set. Our statistical approach is intended to minimize false positive results, particularly with subgroup analysis.
Statistical analysis was performed using SPSS 14.0. In univariate analysis, breast-cancer specific survival (BCSS) was estimated using Kaplan–Meier (KM) curves, and significant differences determined by Log rank tests. For BCSS, survival time was censored at the date of death if the cause was not breast cancer or at the date of last follow-up if the patient was still alive at the end of the study period. 6 patients with unknown cause of death were excluded from BCSS analysis. Cox proportional hazards models were used to calculate adjusted hazard ratios accounting for covariates. Kendall’s tau-b and the Mann-Whitney tests were used to measure the correlation of c-kit status to pathological variables. All statistical tests were two-sided. The Bonferroni correction for multiple comparisons was applied during validation set analysis, and the alpha value for each comparison is 0.05/n, where n is the total number of comparisons.
X-tile analysis
We also used X-tile software [20] to find the optimal cut-off point for the total number of MCs that will predict prognosis in breast cancer patients. X-tile program split the cohort randomly into a matched training and validation set as a method for selecting optimal cut-points. It than calculated a P value for every possible division of the cohort expression data. A two-dimensional graph with its corresponding survival curves was plotted where each colored pixel was proportional to is χ2 Value. The program automatically calculated the maximum χ2 value which served as a cut-point to separate the number of MCs that predicted prognosis.
Results
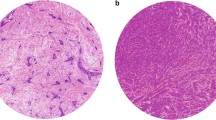
Out of 4,620 cases on the TMAs, we selected 4,444 breast cancers that showed invasive tumor in the cores. The clinico-pathologic characteristics of patients included in the study are depicted in Table 2. The total number of stained MCs was recorded as a continuous variable with counts ranging from 0 to 24 MCs per core. They were seen as 4–20 μm round to oval mononuclear cells with granular cytoplasm and single oval nucleus. The cytoplasmic granules were ganglion-, net-, or crystal-shaped (Fig. 1).
Training set results
Survival analysis
A total of 2,222 patients were included in the training set analysis. After excluding cases which had insufficient invasive tumor, missing core or un-interpretable staining pattern, 1,801 cases were carried forward for the analysis. Out of these, MCs were present in 508 (28.2%) cases. The mean survival time of patients with presence of stromal MCs was 15.0 years (95% CI, 14.5–15.5) compared to 13.9 years (95% CI, 13.5–14.2) for those who did not have positively stained MCs in their tumor stroma. KM survival analysis (Fig. 2a) showed that the presence of stromal MCs was a favourable prognostic marker in the entire training set (BCSS @ 18.4 years, Log rank [Mantel Cox], P = 0.001).
Correlation with other biomarkers
There was positive correlation between MCs and ER (Kendall’s tau-b [τb], 0.034, P = 0.148), Bcl2 (τb = 0.077, P = 0.002), and Her2 (τb = 0.049, P = 0.052), and negative correlation between MCs and EGFR (τb = −0.029, P = 0.228) and CK5/6 (τb = −0.003, P = 0.906) in the training set analysis (Table 3). As these correlations were either not significant or extremely weak, they were not carried forward to the validation set for further analysis.
Nodal status
KM survival analysis showed no statistically significant difference in the survival between tumors with and without MCs in node-negative (BCSS @ 18.1 years, Log rank [Mantel Cox], P = 0.1199) and a significant difference in the node-positive group (BCSS @ 18.3 years, Log rank [Mantel Cox], P = 0.0140). Hence, this result was also not carried forward to the validation set.
Multivariate analysis
Cox proportional hazard model was used to carry out the multivariate analysis and included age, tumor grade, tumor size, nodal status, ER and Her2 as independent predictors of BCSS. All the above variables achieved statistical significance as shown in Table 4(a). Presence of MCs achieved statistical significance (P = 0.041) with a HR = 0.804, 95% CI 0.653–0.991.
Validation set results
Survival analysis
This group included the remaining 2,222 patients from the whole cohort. The mean age at diagnosis was 60 years and the median follow-up was 12.4 years. The median tumor size was 2.0 cm. 50% of patients had Grade 3 tumors, 43% were node positive, and 76% were ER positive. After excluding cases that had insufficient invasive tumor, missing core or un-interpretable staining pattern, 1,796 cases were carried forward for the analysis. Out of these, MCs were present in 494 (27.5%) cases. KM survival analysis (Fig. 2b) showed that the presence of stromal MCs was a favourable prognostic marker in the validation set (P = 0.006).
Multivariate analysis
Cox proportional hazard model was used to carry out the multivariate analysis and included age, tumor grade, tumor size, nodal status, ER and Her2 as independent predictors of BCSS. All the above variables achieved statistical significance as shown in Table 4(b). Presence of MCs did not achieve statistical significance (P = 0.128) but retained a similar HR = 0.846, 95% CI 0.683–1.049 as in the training set analysis.
X-tile analysis results
We assessed the association between patient outcome and number of positively stained MCs in the stroma of tumors using X-tile software. This software allowed us to define an optimal cut-off point that defined the number of MCs needed to predict good prognosis in the cancer patients. The program divided the population into low and high-risk groups based on the number of MCs detected. It converted continuous data into ordinal classes for statistical analysis. The X-tile plot showed that breast cancer patients with any number of stromal MCs had better prognosis compared to those who have no MC infiltration in their stroma. MCs ranging between 1 and 22 in the tumor stroma were present in 507 (27.61%) patients and were grouped together in the low population group of the training set. The high population group comprised of 1,329 (72.39%) patients in the training set. It reiterated the findings obtained by KM survival analysis that the presence of any number of stromal MCs predicts good prognosis in invasive breast cancer patients.
Discussion
The importance of the reciprocal relationship between tumor and stroma is being increasingly recognized [21], and is the role of stromal inflammatory cells like MCs, macrophages, fibroblasts and T cell subtypes in cancer initiation and progression. cDNA microarray analysis has shown that genes expressed by stromal cells correlate with differences in the biology of the tumors and are prognostic predictors in breast cancer [22].
There is an ongoing debate about possible detrimental or beneficial effects of MC accumulation in the stroma of solid tumors. In-vitro studies have shown that MC inhibits tumor growth [23]. This is in agreement with other studies showing increase in MC count in early stage of non-small cell lung cancer and thereby supporting their anti-tumor role. [3]. Increased islet/stromal MC-ratio and presence of tumor islet MCs were shown to be independent good prognostic indicators in non-small-cell lung cancer [24]. Recent studies in ovarian cancer [13] and colorectal cancer [15] have also confirmed their correlation with prognosis.
MCs secrete factors like heparin, interleukin-8 (IL-8) and vascular endothelial growth factor that promote neovascularization; histamine which suppresses immune response; platelet-derived growth factor, nerve growth factor and stem-cell factors which are mitogenic; and proteases that promote metastasis [9]. On the other hand, MCs can inhibit tumor growth by releasing endogenous peroxidase that is cytotoxic to mammalian tumor cells [25], by natural cytotoxicity [21] and by recruiting neutrophils, eosinophils, lymphocytes and macrophages [26]. MCs can secrete protective substances without degranulation in the presence of tumor-derived blockers like oxidized polyamines and thus be detrimental to the tumor [27]. They can destroy tumor cell surface structures directly and indirectly in a fashion similar to the effect of arginase [28]. MCs can also inhibit tumor growth by secreting beneficial cytokines like IL-1, IL-4, IL-6, and tumor necrosis factor-α that induce apoptosis of endothelial cells [29]; and chondroitin sulphate which inhibits metastases [30]. Cathepsin G secreted by MC activates platelets, lymphocytes and macrophages, and is known to be cytotoxic to some mammalian cells [31]. Its inhibition by a tumor cell product was implicated in the progression of advanced squamous cell tumors [32] suggesting its cytotoxic capability against target cells. MCs secrete chymase, which stimulates apoptosis in different target cells [33] and inhibits angiogenesis by exerting cytotoxic effect on vascular smooth muscles [34]. MC tryptase stimulates inflammation and recruits fibroblasts leading to tumor fibrosis, and thereby limiting tumor growth and metastasis [35]. Tryptase, however, is also implicated in angiogenesis and its effect correlated with a better response to chemotherapy in ovarian cancer. This suggests that MC tryptase-mediated fibrosis and angiogenesis may be responsible for the good prognostic effect seen in advanced ovarian cancer [13].
In this TMA study, we showed that the presence of even one or more MCs in the tumor microenvironment was sufficient to exert a positive prognostic effect. Our finding supports the idea of assessing inflammatory cell infiltrates as prognostic markers in cancer. In our previously published pilot report, we had observed no correlation between the presence of stromal MCs with either B-cells (CD20-positive) or T-cells (CD3-positive) [11], and hence we did not stain for these inflammatory cells in this study. We also did not find a statistically significant prognostic effects of MCs in the node-negative group in the training set analysis. This differs from our earlier published report [11] on a 348 case series which concluded that the presence of MCs was a favourable prognostic factor in the node-negative patients (P = 0.018) but not the node-positive group (P = 0.384). This finding underscores the importance of validating clinically relevant findings on a larger series of patients and using the test-validation approach to arrive at a meaningful conclusion.
A number of studies have elaborated on the role of immune cells in cancer etiopathogenesis. T regulatory (Treg) cells are known to be associated with poor outcome in ovarian cancer [36] and the initial results of a clinical trial aimed at specifically eliminating Treg cells has shown promising results [37]. Lymphoma-associated macrophages have been used to predict the outcome of follicular lymphoma [38] and reactive macrophages are used to predict breast, prostate, ovarian, and cervical cancer outcome [39]. Tumor-infiltrating neutrophils are used to predict outcome in patients with adenocarcinoma of the bronchioloalveolar carcinoma subtype [40] and gastric carcinoma [41]. In a recent report, it was concluded that the type, density, and location of immune cells within the tumor samples are a better predictor of patient survival than the histopathological methods currently used to stage colorectal cancer [42].
In conclusion, we confirm our earlier pilot study findings and confirm that stromal MCs correlate with a good prognosis in a large cohort of 4,444 invasive breast cancer patients with long-term follow-up. It highlights the critical role that the host stromal reaction, in particular the inflammatory cell infiltrate, plays in modulating cancer progression. MCs can be used as markers for risk stratification in invasive breast cancers.
References
Baek JY, Tefferi A, Pardanani A, Li CY (2002) Immunohistochemical studies of c-kit, transforming growth factor-beta, and basic fibroblast growth factor in mast cell disease. Leuk Res 26:83–90
Shijubo N, Kojima H, Nagata M, Ohchi T, Suzuki A, Abe S, Sato N (2003) Tumor angiogenesis of non-small cell lung cancer. Microsc Res Tech 60:186-198
Ibaraki T, Muramatsu M, Takai S, Jin D, Maruyama H, Orino T, Katsumata T, Miyazaki M (2005) The relationship of tryptase- and chymase-positive mast cells to angiogenesis in stage I non-small cell lung cancer. Eur J Cardiothorac Surg 28:617–621
Tataroglu C, Kargi A, Ozkal S, Esrefoglu N, Akkoclu A (2004) Association of macrophages, mast cells and eosinophil leukocytes with angiogenesis and tumor stage in non-small cell lung carcinomas (NSCLC). Lung Cancer 43:47–54
Kankkunen JP, Harvima IT, Naukkarinen A (1997) Quantitative analysis of tryptase and chymase containing mast cells in benign and malignant breast lesions. Int J Cancer 72:385–388
Lachter J, Stein M, Lichtig C, Eidelman S, Munichor M (1995) Mast cells in colorectal neoplasias and premalignant disorders. Dis Colon Rectum 38:290–293
Yamamoto T, Katayama I, Nishioka K (1997) Expression of stem cell factor in basal cell carcinoma. Br J Dermatol 137:709–713
Imada A, Shijubo N, Kojima H, Abe S (2000) Mast cells correlate with angiogenesis and poor outcome in stage I lung adenocarcinoma. Eur Respir 15:1087–1093
Theoharides CT, Conti P (2004) Mast cells: the JEKYLL and HYDE of tumor growth. Trends Immunol 25:235–241
Ribatti D, Vacca A, Nico B, Crivellato E, Roncali L, Dammacco F (2001) The role of mast cells in tumour angiogenesis. Br J Haematol 115:514–521
Dabiri S, Huntsman D, Makretsov N, Cheang M, Gilks B, Bajdik C, Gelmon K, Chia S, Hayes M (2004) The presence of stromal mast cells identifies a subset of invasive breast cancers with a favourable prognosis. Mod Pathol 17:690–695
Aaltomaa S, Lipponen P, Papinaho S, Kosma VM (1993) Mast cells in breast cancer. Anticancer Res 13:785–788
Chan JK, Loizzi V, Magistris A, Hunter MI, Rutgers J, DiSaia PJ, Berman ML (2005) Mast cell density, angiogenesis, blood clotting, and prognosis in women with advanced ovarian cancer. Gynecol Oncol 99:20–25
Tomita M, Matsuzaki Y, Onitsuka T (1999) Correlation between mast cells, survival rates in patients with pulmonary adenocarcinoma. Lung Cancer 26:103–108
Tan SY, Fan Y, Luo HS, Shen ZX, Guo Y, Zhao LJ (2005) Prognostic significance of cell infiltrations of immunosurveillance in colorectal cancer. World J Gastroenterol 11:1210–1214
Burtin C, Ponvert C, Fray A, Scheinmann P, Lespinats G, Loridon B, Canu P, Paupe J (1985) Inverse correlation between tumor incidence and tissue histamine levels in W/WV, WV/+, and +/+ mice. J Natl Cancer Inst 74:671–674
Olivotto IA, Bajdik CD, Ravdin PM, Speers CH, Coldman AJ, Norris BD, Davis GJ, Chia SK, Gelmon KA (2005) Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol 23:2716–2725
Cheang MC, Treaba DO, Speers CH, Olivotto IA, Bajdik CD, Chia SK, Goldstein LC, Gelmon KA, Huntsman D, Gilks CB, Nielsen TO, Gown AM (2006) Immunohistochemical detection using the new rabbit monoclonal antibody SP1 of estrogen receptor in breast cancer is superior to mouse monoclonal antibody 1D5 in predicting survival. J Clin Oncol 24:5637–5644
Liu CL, Prapong W, Natkunam Y, Alizadeh A, Montgomery K, Gilks CB, van de Rijn M (2002) Software tools for high-throughput analysis and archiving of immunohistochemistry staining data obtained with tissue microarrays. Am J Pathol 161:1557–1565
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10:7252–7259
Bhowmick AN, Moses LH (2005) Tumor-stroma interactions. Curr Opin Genet Dev 15:97–101
West RB, Nuyten DS, Subramanian S, Nielsen TO, Corless CL, Rubin BP, Montgomery K, Zhu S, Patel R, Hernandez-Boussard T, Goldblum JR, Brown PO, van de Vijver M, van de Rijn M (2005) Determination of stromal signatures in breast carcinoma. PLoS Biol 3:e187
Ghiara P, Boraschi D, Villa L, Scapigliati G, Taddei C, Tagliabue (1985) In vitro generated mast cells express natural cytotoxicity against tumour cells. Immunology 55:317–324
Welsh TJ, Green RH, Richardson D, Waller DA, O’Byrne KJ, Bradding P (2005) Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol 23:8959–8967
Henderson WR, Chi EY, Jong EC, Klebanoff SJ (1981) Mast cell-mediated tumor-cell cytotoxicity. Role of the peroxidase system. J Exp Med 153:520–533
He S, Walls AF (1998) Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo. Br J Pharmacol 125:1491–1500
Vliagoftis H, Boucher WS, Mak LL, Theoharides CT (1992) Inhibition of mast cell secretion by oxidation products of natural polyamines. Biochem Pharmacol 43:2237–2245
Farram E, Nelson DS (1980) Mouse mast cells as anti-tumor effector cells. Cell Immunol 55:294–301
Latti S, Leskinen M, Shiota N, Wang Y, Kovanen PT, Lindstedt KA (2003) Mast cell-mediated apoptosis of endothelial cells in vitro: a paracrine mechanism involving TNF-alpha-mediated down-regulation of bcl-2 expression. J Cell Physiol 195:130–138
Kokenyesi R (2001) Ovarian carcinoma cells synthesize both chondroitin sulfate and heparan sulfate cell surface proteoglycans that mediate cell adhesion to interstitial matrix. J Cell Biochem 83:259–270
Johnson RJ, Couser WG, Alpers CE, Vissers M, Schulze M, Klebanoff SJ (1988) The human neutrophil serine proteinases, elastase and cathepsin G, can mediate glomerular injury in vivo. J Exp Med 168:1169–1174
Schick C, Kamachi Y, Bartuski AJ, Cataltepe S, Schechter NM, Pemberton PA, Silverman GA (1997) Squamous cell carcinoma antigen 2 is a novel serpin that inhibits the chymotrypsin-like proteinases cathepsin G and mast cell chymase. J Biol Chem 272:1849–1855
Hara M, Matsumori A, Ono K, Kido H, Hwang MW, Miyamoto T, Iwasaki A, Okada M, Nakatani K, Sasayama S (1999) Mast cells cause apoptosis of cardiomyocytes and proliferation of other intramyocardial cells in vitro. Circulation 100:1443–1449
Leskinen MJ, Lindstedt KA, Wang Y, Kovanen PT (2003) Mast cell chymase induces smooth muscle cell apoptosis by a mechanism involving fibronectin degradation and disruption of focal adhesions. Arterioscler Thromb Vasc Biol 23:238–243
Ruoss SJ, Hartmann T, Caughey GH (1991) Mast cell tryptase is a mitogen for cultured fibroblasts. J Clin Invest 88:493–499
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949
Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J (2005) Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 115:3623–3633
Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, Klasa R, Voss N, Connors JM, Gascoyne RD (2005) Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood 106:2169–2174
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4:71–78
Bellocq A, Antoine M, Flahault A, Philippe C, Crestani B, Bernaudin JF, Mayaud C, Milleron B, Baud L, Cadranel J (1998) Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol 152:83–92
Caruso RA, Bellocco R, Pagano M, Bertoli G, Rigoli L, Inferrera C (2002) Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in northern Italy. Mod Pathol 15:831–837
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Acknowledgements
This work was supported in part by the Canadian Institutes of Health Research Strategic Training Program (Grant STP-53912), Translation Acceleration Grant from the Canadian Breast Cancer Research Alliance and the National Institute of Health Strategic Partnering to Evaluate Cancer Signatures program. The Genetic Pathology Evaluation Center is supported by an unrestricted educational grant from sanofi-aventis, Canada. D. Huntsman and T. Nielsen are Michael Smith Health Foundation for Research Scholars.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 2.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by-nc/2.0/.
About this article
Cite this article
Rajput, A.B., Turbin, D.A., Cheang, M.C. et al. Stromal mast cells in invasive breast cancer are a marker of favourable prognosis: a study of 4,444 cases. Breast Cancer Res Treat 107, 249–257 (2008). https://doi.org/10.1007/s10549-007-9546-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9546-3