Abstract
Background
The two-gene expression ratio HOXB13:IL17BR has been proposed to predict the outcome of tamoxifen-treated breast cancer patients. We intended to examine whether this ratio can predict the benefit of 5 years vs. 2 years of tamoxifen treatment of postmenopausal patients. A further objective was to investigate any prognostic effects of the ratio in systematically untreated premenopausal patients. Based on the current knowledge of HOXB13 and IL17BR, we hypothesized that these genes may have individual prognostic or predictive power.
Patients and methods
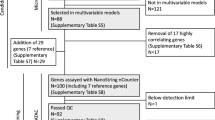
Expression of HOXB13 and IL17BR were quantified by real-time PCR in tumors from 264 randomized postmenopausal patients and 93 systemically untreated premenopausal patients.
Results
A high HOXB13:IL17BR ratio was associated with aggressive tumor characteristics, as were low levels of IL17BR alone. The ratio and HOXB13 alone predicted recurrence-free survival after endocrine treatment, with a benefit of prolonged treatment in estrogen receptor-positive patients correlated to a low ratio (recurrence rate ratio: RR = 0.39; P = 0.030), or low expression of HOXB13 (RR = 0.37; P = 0.015). No difference in recurrence-free survival was seen for the high ratio or high HOXB13 subgroups. The predictive value of HOXB13 and HOXB13:IL17BR was significant in multivariate analysis. In the systemically untreated cohort, only IL17BR showed independent prognostic significance.
Conclusion
We conclude that the ratio or HOXB13 alone can predict the benefit of endocrine therapy, with a high ratio or a high expression rendering patients less likely to respond. We have also shown that IL17BR might be an independent prognostic factor in breast cancer.


Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717
Sorlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874
Gruvberger S, Ringner M, Chen Y et al (2001) Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res 61(16):5979–5984
Sotiriou C, Neo SY, McShane LM et al (2003) Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA 100(18):10393–10398
van ‘t Veer LJ, Dai H, van de Vijver MJ et al (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415(6871):530–536
Wang Y, Klijn JG, Zhang Y et al (2005) Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 365(9460):671–679
van de Vijver MJ, He YD, van ‘t Veer LJ et al (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347(25):1999–2009
Jansen MP, Foekens JA, van Staveren IL et al (2005) Molecular classification of tamoxifen-resistant breast carcinomas by gene expression profiling. J Clin Oncol 23(4):732–740
Paik S, Shak S, Tang G et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826
Ma XJ, Wang Z, Ryan PD et al (2004) A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell 5(6):607–616
Reid JF, Lusa L, De Cecco L et al (2005) Limits of predictive models using microarray data for breast cancer clinical treatment outcome. J Natl Cancer Inst 97(12):927–930
Goetz MP, Suman VJ, Ingle JN et al (2006) A two-gene expression ratio of homeobox 13 and interleukin−17B receptor for prediction of recurrence and survival in women receiving adjuvant tamoxifen. Clin Cancer Res 12(7 Pt 1):2080–2087
Ma XJ, Hilsenbeck SG, Wang W et al (2006) The HOXB13:IL17BR expression index is a prognostic factor in early-stage breast cancer. J Clin Oncol 24(28):4611–4619
Swedish Breast Cancer Cooperative Group (1996) Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. J Natl Cancer Inst 88(21):1543–1549
Ryden L, Jonsson PE, Chebil G, et al (2005) Two years of adjuvant tamoxifen in premenopausal patients with breast cancer: a randomised, controlled trial with long-term follow-up. Eur J Cancer 41(2):256–264
Fernö M, Stål O, Baldetorp B et al (2000) Results of two or five years of adjuvant tamoxifen correlated to steroid receptor and S-phase levels. South Sweden Breast Cancer Group, and South–East Sweden Breast Cancer Group. Breast Cancer Res Treat 59(1):69–76
Stål O, Borg Å, Fernö M et al (2000) ErbB2 status and the benefit from two or five years of adjuvant tamoxifen in postmenopausal early stage breast cancer. Ann Oncol 11(12):1545–1550
Edwards S, Campbell C, Flohr P, et al (2005) Expression analysis onto microarrays of randomly selected cDNA clones highlights HOXB13 as a marker of human prostate cancer. Br J Cancer 92(2):376–381
Zhao Y, Yamashita T, Ishikawa M (2005) Regulation of tumor invasion by HOXB13 gene overexpressed in human endometrial cancer. Oncol Rep 13(4):721–726
Lopez R, Garrido E, Pina P et al (2006) HOXB homeobox gene expression in cervical carcinoma. Int J Gynecol Cancer 16(1):329–335
Okuda H, Toyota M, Ishida W, et al (2006) Epigenetic inactivation of the candidate tumor suppressor gene HOXB13 in human renal cell carcinoma. Oncogene 25(12):1733–1742
Jung C, Kim RS, Zhang H et al (2005) HOXB13 is downregulated in colorectal cancer to confer TCF4-mediated transactivation. Br J Cancer 92(12):2233–2239
Maeda K, Hamada J, Takahashi Y, et al (2005) Altered expressions of HOX genes in human cutaneous malignant melanoma. Int J Cancer 114(3):436–441
Jung C, Kim RS, Lee SJ et al (2004) HOXB13 homeodomain protein suppresses the growth of prostate cancer cells by the negative regulation of T-cell factor 4. Cancer Res 64(9):3046–3051
Jung C, Kim RS, Zhang HJ et al (2004) HOXB13 induces growth suppression of prostate cancer cells as a repressor of hormone-activated androgen receptor signaling. Cancer Res 64(24):9185–9192
Rodriguez BAT, Jin VX, Cheng AS, et al (2006) HOXB13 is an estrogen receptor responsive gene preferentially methylated in ER-positive breast cancer. In: Proceedings of the 97th annual meeting, AACR, Washington, USA, 1–5 April 2006
Cantile M, Pettinato G, Procino A, et al (2003) In vivo expression of the whole HOX gene network in human breast cancer. Eur J Cancer 39(2):257–264
Apiou F, Flagiello D, Cillo C, et al (1996) Fine mapping of human HOX gene clusters. Cytogenet Cell Genet 73(1–2):114–115
Shi Y, Ullrich SJ, Zhang J, et al (2000) A novel cytokine receptor–ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J Biol Chem 275(25):19167–19176
Lee J, Ho WH, Maruoka M, et al (2001) IL−17E, a novel proinflammatory ligand for the IL−17 receptor homolog IL−17Rh1. J Biol Chem 276(2):1660–1664
Acknowledgements
We are indebted to participating members of the South and Southeast Sweden Breast Cancer Groups for providing us with breast cancer samples and to Gunilla Chebil and Sten Thorstenson for re-evaluation of histological grade. This study was supported by funds from the Swedish Cancer Society, the Gunnar Arvid and Elisabeth Nilsson Foundation, the Mrs Berta Kamprad Foundation, the Swedish Research Council and the University Hospital of Lund Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Piiha-Lotta Jerevall, Sara Brommesson contributed equally.
Mårten Fernö, Olle Stål—Equally shared senior authorship
Rights and permissions
About this article
Cite this article
Jerevall, PL., Brommesson, S., Strand, C. et al. Exploring the two-gene ratio in breast cancer—independent roles for HOXB13 and IL17BR in prediction of clinical outcome. Breast Cancer Res Treat 107, 225–234 (2008). https://doi.org/10.1007/s10549-007-9541-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9541-8