Abstract
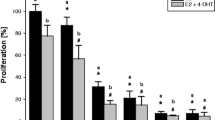
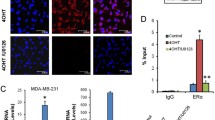
Response to treatment with the antiestrogen tamoxifen is variable and at least partially due to its highly complex metabolism. Tamoxifen is transformed by polymorphic and inducible cytochrome P450 enzymes to a large number of metabolites with varying biological activities. The estrogen receptor dependent growth inhibitory effect of antiestrogens is mediated by activation of antiproliferative Transforming Growth Factor beta (TGFβ) signal transduction pathways. The aim of the present study was to establish if TGFβ2 or TGFβ receptor II (TβRII), could be used as markers to assess the pharmacological potency of tamoxifen and its metabolites. Consequently, we analyzed the growth inhibitory effect of tamoxifen and its major metabolites and explored whether it correlated with their capacity to induce TGFβ2 and TβRII expression. Human breast cancer cells (MCF-7 and T47D) were treated with tamoxifen and tamoxifen metabolites and mRNA expression of TGFβ2 and TβRII was analyzed by quantitative RT-PCR. Only two metabolites 4-hydroxytamoxifen and N-desmethyl-4-hydroxytamoxifen had significant antiproliferative activity and were able to induce TGFβ2 and TβRII. Plasma concentrations of these metabolites are usually very low in patients. However, even minor growth inhibitory effects at concentrations which are below the limit of quantification in plasma samples resulted in clearly discernible effects on expression of TGFβ2 and TβRII. Taken together, our data demonstrate that TGFβ2 and TβRII are very specific and sensitive biomarkers for the antiestrogenic activity of tamoxifen metabolites in breast cancer.





Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
Boocock DJ, Brown K, Gibbs AH et al (2002) Identification of human CYP forms involved in the activation of tamoxifen and irreversible binding to DNA. Carcinogenesis 23:1897–1901
Coller JK, Krebsfaenger N, Klein K et al (2002) The influence of CYP2B6, CYP2C9 and CYP2D6 genotypes on the formation of the potent antioestrogen Z-4-hydroxy-tamoxifen in human liver. Br J Clin Pharmacol 54:157–167
Coller JK, Krebsfaenger N, Klein K et al (2004) Large interindividual variability in the in vitro formation of tamoxifen metabolites related to the development of genotoxicity. Br J Clin Pharmacol 57:105–111
Crewe HK, Ellis SW, Lennard MS, Tucker GT (1997) Variable contribution of cytochromes P450 2D6, 2C9 and 3A4 to the 4-hydroxylation of tamoxifen by human liver microsomes. Biochem Pharmacol 53:171–178
Desta Z, Ward BA, Soukhova NV, Flockhart DA (2004) Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 310:1062–1075
Mani C, Gelboin HV, Park SS et al (1993) Metabolism of the antimammary cancer antiestrogenic agent tamoxifen. I. Cytochrome P-450-catalyzed N-demethylation and 4-hydroxylation. Drug Metab Dispos 21:645–656
Parte P, Kupfer D (2005) Oxidation of tamoxifen by human flavin-containing monooxygenase (FMO) 1 and FMO3 to tamoxifen-N-oxide and its novel reduction back to tamoxifen by human cytochromes P450 and hemoglobin. Drug Metab Dispos 33:1446–1452
Kivisto KT, Villikka K, Nyman L et al (1998) Tamoxifen and toremifene concentrations in plasma are greatly decreased by rifampin. Clin Pharmacol Ther 64:648–654
Lien EA, Anker G, Lonning PE et al (1990) Decreased serum concentrations of tamoxifen and its metabolites induced by aminoglutethimide. Cancer Res 50:5851–5857
Stearns V, Johnson MD, Rae JM et al (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95:1758–1764
Coezy E, Borgna JL, Rochefort H (1982) Tamoxifen and metabolites in MCF7 cells: correlation between binding to estrogen receptor and inhibition of cell growth. Cancer Res 42:317–323
Etienne MC, Milano G, Fischel JL et al (1989) Tamoxifen metabolism: pharmacokinetic and in vitro study. Br J Cancer 60:30–35
Jordan VC, Collins MM, Rowsby L, Prestwich G (1977) A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol 75:305–316
Johnson MD, Zuo H, Lee KH et al (2004) Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 85:151–159
Lien EA, Solheim E, Lea OA et al (1989) Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res 49:2175–2183
Lien EA, Solheim E, Ueland PM (1991) Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res 51:4837–4844
Knabbe C, Kopp A, Hilgers W et al (1996) Regulation and role of TGF beta production in breast cancer. Ann N Y Acad Sci 784:263–276
Zugmaier G, Ennis BW, Deschauer B et al (1989) Transforming growth factors type beta 1 and beta 2 are equipotent growth inhibitors of human breast cancer cell lines. J Cell Physiol 141:353–361
Moustakas A, Heldin CH (2005) Non-Smad TGF-beta signals. J Cell Sci 118:3573–3584
Brandt S, Kopp A, Grage B et al (2003) Effects of tamoxifen on transcriptional level of transforming growth factor beta (TGF-beta) isoforms 1 and 2 in tumor tissue during primary treatment of patients with breast cancer. Anticancer Res 23:223–229
Buck MB, Pfizenmaier K, Knabbe C (2004) Antiestrogens induce growth inhibition by sequential activation of p38 mitogen-activated protein kinase and transforming growth factor-beta pathways in human breast cancer cells. Mol Endocrinol 18:1643–1657
Muller V, Jensen EV, Knabbe C (1998) Partial antagonism between steroidal and nonsteroidal antiestrogens in human breast cancer cell lines. Cancer Res 58:263–267
Knabbe C, Zugmaier G, Schmahl M et al (1991) Induction of transforming growth factor beta by the antiestrogens droloxifene, tamoxifen, and toremifene in MCF-7 cells. Am J Clin Oncol 14 Suppl 2:S15–S20
Kopp A, Jonat W, Schmahl M, Knabbe C (1995) Transforming growth factor beta 2 (TGF-beta 2) levels in plasma of patients with metastatic breast cancer treated with tamoxifen. Cancer Res 55:4512–4515
Gauthier S, Mailhot J, Labrie F (1996) New highly stereoselective synthesis of (Z)-4-hydroxytamoxifen and (Z)-4-hydroxytoremifene via McMurry reaction. J Org Chem 61:3890–3893
Coradini D, Cappelletti V, Granata G et al(1991) Activity of tamoxifen and its metabolites on endocrine-dependent and endocrine-independent breast cancer cells. Tumour Biol 12:149–158
Taylor CM, Blanchard B, Zava DT (1984) Estrogen receptor-mediated and cytotoxic effects of the antiestrogens tamoxifen and 4-hydroxytamoxifen. Cancer Res 44:1409–1414
Fabian C, Tilzer L, Sternson L (1981) Comparative binding affinities of tamoxifen, 4-hydroxytamoxifen, and desmethyltamoxifen for estrogen receptors isolated from human breast carcinoma: correlation with blood levels in patients with metastatic breast cancer. Biopharm Drug Dispos 2:381–390
Kemp JV, Adam HK, Wakeling AE, Slater R (1983) Identification and biological activity of tamoxifen metabolites in human serum. Biochem Pharmacol 32:2045–2052
Langan-Fahey SM, Tormey DC, Jordan VC (1990) Tamoxifen metabolites in patients on long-term adjuvant therapy for breast cancer. Eur J Cancer 26:883–888
MacCallum J, Cummings J, Dixon JM, Miller WR (2000) Concentrations of tamoxifen and its major metabolites in hormone responsive and resistant breast tumours. Br J Cancer 82:1629–1635
Milano G, Etienne MC, Frenay M et al (1987) Optimised analysis of tamoxifen and its main metabolites in the plasma and cytosol of mammary tumours. Br J Cancer 55:509–512
Murphy C, Fotsis T, Pantzar P et al (1987) Analysis of tamoxifen, N-desmethyltamoxifen and 4-hydroxytamoxifen levels in cytosol and KCl-nuclear extracts of breast tumours from tamoxifen treated patients by gas chromatography-mass spectrometry (GC-MS) using selected ion monitoring (SIM). J Steroid Biochem 28:609–618
Crewe HK, Notley LM, Wunsch RM et al (2002) Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4′-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos 30:869–874
Zanger UM, Raimundo S, Eichelbaum M (2004) Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol 369:23–37
Jin Y, Desta Z, Stearns V et al (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97:30–39
Dresser GK, Bailey DG (2002) A basic conceptual and practical overview of interactions with highly prescribed drugs. Can J Clin Pharmacol 9:191–198
Kivisto KT, Kroemer HK, Eichelbaum M (1995) The role of human cytochrome P450 enzymes in the metabolism of anticancer agents: implications for drug interactions. Br J Clin Pharmacol 40:523–530
Acknowledgments
This work was supported by the Robert Bosch Foundation. JK Coller was a recipient of a CJ Martin Postdoctoral Training Fellowship from the National Health and Medical Research Council (NHMRC) of Australia.
We thank Stefanie Laukemann and Tabea Peußer for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buck, M.B., Coller, J.K., Mürdter, T.E. et al. TGFβ2 and TβRII are valid molecular biomarkers for the antiproliferative effects of tamoxifen and tamoxifen metabolites in breast cancer cells. Breast Cancer Res Treat 107, 15–24 (2008). https://doi.org/10.1007/s10549-007-9526-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9526-7