Abstract
Purpose
Development of new capillary blood vessels is essential for the growth of cancer. Two distinct processes, vasculogenesis and angiogenesis implement the formation of the new vascular network. Recently, it was demonstrated that vasculogenesis creates the primary network of vascular endothelial cells that will become major blood vessels in malignant tumors by the recruitment of CD34+/vascular endothelial growth factor receptor 2 (FLK-1)+ endothelial progenitor cells (EPCs) to sites of the new vessel formation with subsequent differentiation into mature endothelial cell. Therefore the aim of this study was a) to quantitate EPCs in breast cancer patients and b) to evaluate if the release of EPCs into the circulation is mainly regulated by the tumor himself.
Experimental design
CD34+FLK-1+ EPCs were measured in the peripheral circulation of patients with breast cancer (n = 47) before and after therapy. Furthermore the potential of EPCs to differentiate into endothelial cells was investigated by late-outgrowth experiments and the metabolic uptake of dil-acetylated-LDL and immunoreactivity against von Willebrand factor.
Results
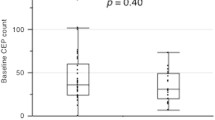
In breast cancer patients the amount of CD34+FLK-1+ EPCs (percent of peripheral blood mononuclear cells) is significantly increased in women with breast cancer. Tumors larger than 2 cm showed significantly higher values of CD34+FLK-1+ EPCs. After excision of the tumor the amount of CD34+FLK-1+ EPCs rapidly declines.
Conclusions
Our findings lead to the tumor, as source of angiogenic chemokines, is most important for recruiting CD34+FLK-1+ EPCs during breast cancer development. Therefore circulating endothelial progenitor cells may work as a new diagnostic tool in the screening and diagnosis of breast cancer.




Similar content being viewed by others
References
Goldmann E (1907) The growth of malignant disease in man and the lower animals with special reference to the vascular system. Lancet 2:1236–1240
Ide A, Baker NH, Warren SL (1939) Vascularization of the Brown-Pearce rabbit epithelioma transplant as seen in the transparent ear chamber. Am J Radiol 42:891–899
Algire GH, Chalkley HW (1945) Vascular reactions of normal and malignant tissues in vivo. I. Vascular reactions of mice to wounds and to normal and neoplastic transplants. J Natl Cancer Inst USA 6:73–85
Folkman J (2000) In: Holland JF et al (eds) Cancer medicine. Decker, Ontario, Canada, pp 132–152
Asahara T, Masuda H, Takahashi T et al (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularisation. Circ Res 85:221–228
Gehling UM, Ergün S, Schumacher U et al (2000) In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood 95:3106–3112
Lyden D, Hattori K, Dias S et al (2001) Impaired recruitment of bone-marrow-derived endothelial and haematopoetic precursor cells blocks tumor angiogenesis and growth. Nature Med 7:1194–1201
Reyes M, Dudek A, Jahagirgar B et al (2002) Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest 109:337–346
Asahara T, Takahashi T, Masuda H et al (1999) VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18:3964–3972
Shi Q, Rafii S, Wu MHD et al (1998) Evidence for circulating bone marrow-derived endothelial cells. Blood 92:362–367
Asahara T, Murohara T, Sullivan A et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967
Lin Y, Weisdorf D, Solovey A, et al (2000) Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest 105:71–77
Gill M, Dias S, Hattori K et al (2001) Vascular Trauma Induces Rapid but Transient Mobilization of VEGFR2+ AC133+ Endothelial Precursor Cells. Circ Res 88:167–174
Peichev M, Naiyer A, Pereira D et al (2000) Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood 95:952–958
Quirici N, Soligo D, Caneva L et al (2001) Differentiation and expansion of endothelial cells from human bone marrow CD133+ cells. Br J Haematol 115:186–194
Hristov M, Erl W, Weber P (2003) Endothelial progenitor cells. Mobilization differentiation, and homing. Arterioscler Thromb Vasc Biol 23:1–6
Rafii S (2000) Circulating endothelial precursors: mystery, reality, and promise. J Clin Invest 105:17–19
Lyden D, Young A, Zagzag D et al (1999) Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401:670–677
Carmeliet P, Moons L, Luttun A et al (2001) Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 7:575–583
Rafii S, Lyden D, Benezra R et al (2002) Vascular and haematopoietic stem cells: Novel targets for anti-angiogenesis therapy? Nature Cancer Rev 2:826–835
Bergers G, Brekken R, McMahon G et al (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature Cell Biololy 2:737–744
Heissig B, Hattori K, Dias S et al (2002) Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9mediated release of Kit-ligand. Cell 109:625–637
Bertolini F, Paul S, Mancuso P et al (2003) Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 63:4342–4346
Kraft A, Weindel K, Ochs A et al (1999) Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer 85:178–187
Yamamoto Y, Toi M, Kondo S et al (1996) Concentrations of vascular endothelial growth factor in sera of normal controls and cancer patients. Clin Cancer Res 2:821–826
Acknowledgements
None of the authors received financial support or a grant for this study
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Richter-Ehrenstein, C., Rentzsch, J., Runkel, S. et al. Endothelial progenitor cells in breast cancer patients. Breast Cancer Res Treat 106, 343–349 (2007). https://doi.org/10.1007/s10549-007-9505-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9505-z