Abstract
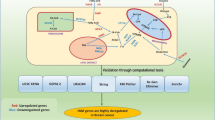
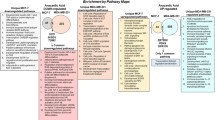
Essential fatty acids have long been identified as possible oncogenic factors. Existing reports suggest omega-6 (ω-6) essential fatty acids (EFA) as pro-oncogenic and omega-3 (ω-3) EFA as anti-oncogenic factors. The ω-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), inhibit the growth of human breast cancer cells while the ω-6 fatty acids induces growth of these cells in animal models and cell lines. In order to explore likely mechanisms for the modulation of breast cancer cell growth by ω-3 and ω-6 fatty acids, we examined the effects of arachidonic acid (AA), linoleic Acid (LA), EPA and DHA on human breast cancer cell lines using cDNA microarrays and quantitative polymerase chain reaction. MDA-MB-231, MDA-MB-435s, MCF-7 and HCC2218 cell lines were treated with the selected fatty acids for 6 and 24 h. Microarray analysis of gene expression profiles in the breast cancer cells treated with both classes of fatty acids discerned essential differences among the two classes at the earlier time point. The differential effects of ω-3 and ω-6 fatty acids on the breast cancer cells were lessened at the late time point. Data mining and statistical analyses identified genes that were differentially expressed between breast cancer cells treated with ω-3 and ω-6 fatty acids. Ontological investigations have associated those genes to a broad spectrum of biological functions, including cellular nutrition, cell division, cell proliferation, metastasis and transcription factors etc., and thus presented an important pool of biomarkers for the differential effect of ω-3 and ω-6EFAs.
Similar content being viewed by others
References
Apantaku LM (2000) Breast cancer diagnosis and screening. Am Fam Physician 62(3):596–602, 605–606
Apantaku LM (2002) Breast-conserving surgery for breast cancer. Am Fam Physician 66(12): 2271–2278
Carroll KK (1985) Dietary fat in relation to mammary carcinogenesis. Princess Takamatsu Symp 16:255–263
Enig MG, Munn RJ, Keeney M (1978) Dietary fat and cancer trends–a critique. Fed Proc 37(9):2215–2220
Hilakivi-Clarke L, Olivo SE, Shajahan A, Khan G, Zhu Y, Zwart A, Cho E, Clarke R (2005) Mechanisms mediating the effects of prepubertal (n–3) polyunsaturated fatty acid diet on breast cancer risk in rats. J Nutr 135(12 Suppl):2946S–2952S
Schley PD, Jijon HB, Robinson LE, Field CJ (2005) Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat 92(2):187–195
Wu M, Harvey KA, Ruzmetov N, Welch ZR, Sech L, Jackson K, Stillwell W, Zaloga GP, Siddiqui RA (2005) Omega-3 polyunsaturated fatty acids attenuate breast cancer growth through activation of a neutral sphingomyelinase-mediated pathway. Int J Cancer 117(3):340–348
Cohen LA (1997) Breast cancer risk in rats fed a diet high in n–6 polyunsaturated fatty acids during pregnancy. J Natl Cancer Inst 89(9):662–663
Lanson M, Bougnoux P, Besson P, Lansac J, Hubert B, Couet C, Le Floch O (1990) N–6 polyunsaturated fatty acids in human breast carcinoma phosphatidylethanolamine and early relapse. Br J Cancer 61(5):776–778
Chajes V, Sattler W, Stranzl A, Kostner GM (1995) Influence of n–3 fatty acids on the growth of human breast cancer cells in vitro: relationship to peroxides and vitamin-E. Breast Cancer Res Treat 34(3):199–212
Karmali RA (1989) n–3 fatty acids and cancer. J Intern Med Suppl 731:197–200
Grammatikos SI, Subbaiah PV, Victor TA, Miller WM (1994) n–3 and n–6 fatty acid processing and growth effects in neoplastic and non-cancerous human mammary epithelial cell lines. Br J Cancer 70(2):219–227
Hammamieh R, Chakraborty N, Das R, Jett M (2004) Molecular impacts of antisense complementary to the liver fatty acid binding protein (FABP) mRNA in DU 145 prostate cancer cells in vitro. J Exp Ther Oncol 4(3):195–202
Esmon CT (2003) The protein C pathway. Chest 124(3 Suppl):26S–32S
Yin Y, Liu YX, Jin YJ, Hall EJ, Barrett JC (2003) PAC1 phosphatase is a transcription target of p53 in signalling apoptosis and growth suppression. Nature 422(6931): 527–531
Germani A, Prabel A, Mourah S, Podgorniak MP, Di Carlo A, Ehrlich R, Gisselbrecht S, Varin-Blank N, Calvo F, Bruzzoni-Giovanelli H (2003) SIAH-1 interacts with CtIP and promotes its degradation by the proteasome pathway. Oncogene 22(55):8845–8851
Chiyo M, Shimozato O, Yu L, Kawamura K, Iizasa T, Fujisawa T, Tagawa M (2005) Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer 115(3):437–442
Zhang Y, Pasparakis M, Kollias G, Simons M (1999) Myocyte-dependent regulation of endothelial cell syndecan-4 expression. Role of TNF-alpha. J Biol Chem 274(21):14,786–14,790
Lee BP, Rushlow WJ, Chakraborty C, Lala PK (2001) Differential gene expression in premalignant human trophoblast: role of IGFBP-5. Int J Cancer 94(5):674–684
Jensen LE, Whitehead AS (2003) Expression of alternatively spliced interleukin-1 receptor accessory protein mRNAs is differentially regulated during inflammation and apoptosis. Cell Signal 15(8):793–802
Nagahata T, Sato T, Tomura A, Onda M, Nishikawa K, Emi M (2005) Identification of RAI3 as a therapeutic target for breast cancer. Endocr Relat Cancer 12(1):65–73
Pigott DA, Grimaldi MA, Dell’Aquila ML, Gaffney EV (1982) Growth inhibitors in plasma derived human serum. In Vitro 18(7):617–625
Cardoso F, Ross JS, Picart MJ, Sotiriou C, Durbecq V (2004) Targeting the ubiquitin-proteasome pathway in breast cancer. Clin Breast Cancer 5(2):148–157
Cloitre M, Heimberg RG, Holt CS, Liebowitz MR (1992) Reaction time to threat stimuli in panic disorder and social phobia. Behav Res Ther 30(6):609–617
Dennis JW, Laferte S, Yagel S, Breitman ML (1989) Asparagine-linked oligosaccharides associated with metastatic cancer. Cancer Cells 1(3):87–92
Xiang Q, Fan SQ, Li J, Tan C, Xiang JJ, Zhang QH, Wang R, Li GY (2002) [Expression of connexin43 and connexin45 in nasopharyngeal carcinoma]. Ai Zheng 21(6):593–596
Hodgson JG, Malek T, Bornstein S, Hariono S, Ginzinger DG, Muller WJ, Gray JW (2005) Copy number aberrations in mouse breast tumors reveal loci and genes important in tumorigenic receptor tyrosine kinase signaling. Cancer Res 65(21):9695–9704
Yokozaki H, Budillon A, Tortora G, Meissner S, Beaucage SL, Miki K, Cho-Chung YS (1993) An antisense oligodeoxynucleotide that depletes RI alpha subunit of cyclic AMP-dependent protein kinase induces growth inhibition in human cancer cells. Cancer Res 53(4):868–872
Acknowledgements
This work is partially funded by a grant from the US Army Medical Research and Materiel Command, award number DAMD-17-01-1-0268
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Hammamieh and N. Chakraborty contributed equally to this manuscript
Rights and permissions
About this article
Cite this article
Hammamieh, R., Chakraborty, N., Miller, SA. et al. Differential Effects of Omega-3 and Omega-6 fatty Acids on Gene Expression in Breast Cancer Cells. Breast Cancer Res Treat 101, 7–16 (2007). https://doi.org/10.1007/s10549-006-9269-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9269-x