Summary
Purpose
We are conducting clinical trials in breast cancer (BrCa) patients to test the HER2/neu peptide vaccine (E75). We have investigated the impact of this vaccine on circulating levels of regulatory T cells (Treg) and the resulting effects on antitumor responses.
Experimental design
Twenty-two blood samples from healthy individuals and from 22 BrCa patients including pre- and post-vaccination samples from seven vaccinated HLA-A2+ patients were stained for CD4, CD25, and CD69 as well as CD8 and E75:HLA-A2 Ig dimer and quantified by flow cytometry. Cytotoxic activity against HER2/neu + tumors was measured by 51Cr-release. Serum from BrCa patients and normal subjects were analyzed for TGF-β levels.
Results
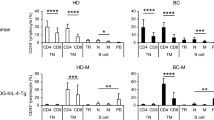
BrCa patients have a greater percentage of circulating Treg (CD4+CD25+, 4.45% versus 2.96%; p = 0.007) than normal subjects. HLA-A2+ BrCa patients had more Treg compared to the HLA-A2– BrCa patients (CD4+CD25+, 5.63% versus 3.28%; p = 0.001). E75 vaccination increased circulating activated CD4+ T cells post-vaccination (CD4+CD69+, 1.23 versus 3.81%; p = 0.03). However, Treg were significantly reduced after vaccination (CD4+CD25+, 5.31–1.81%; p < 0.0001). Furthermore, activated Treg also decreased (CD4+CD25+CD69+, 0.23% versus 0.08%; p = 0.06). Importantly, post-vaccination decreases in Treg were temporally associated with increased E75 vaccine-specific CD8+ T cells and corresponding HER2/neu + tumor cytotoxicity. Serum TGF-β levels were significantly elevated in BrCa patients compared to normals (3548 pg/ml versus 1007 pg/ml; p = 0.007). Four of seven vaccinated patients showed decreased serum TGF-β levels post-vaccination.
Conclusions
Treg, are increased in BrCa patients along with serum levels of TGF-β. E75 vaccination resulted in CD4+ recruitment but was associated with a significant decrease in circulating Treg and TGF-β levels in the majority of the vaccinated patients. Successful cancer vaccination strategies may require the alteration of complex immune interactions.
Similar content being viewed by others
References
Dorsch S, Roser R, Recirculating, suppressor T cells in transplantation toleranceJ Exp Med 145: 1144–1157, 1977
Coutinho A, Hori S, Carvalho T, Caramalho I, Demengeot J, Regulatory T cells: the physiology of autoreactivity in dominant tolerance and “quality control” of immune responsesImmunol Rev182: 89–98, 2001
Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T, Organ-specific autoimmune diseases induced in mice by elimination of T cell subset: I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune diseaseJ Exp Med 161: 72–87, 1985
Tung KSK, Smith S, Teuscher C, Cook C, Anderson RE, Murine autoimmune oophoritis, epididymoorchitis, and gastritis induced by day 3 thymectomy: immunopathologyAm J Pathol 126: 293–302, 1987
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M, Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25): breakdown of a single mechanism of self-tolerance causes autoimmune diseasesJ Immunol 155: 1151–1164, 1995
Asano M, Toda M, Sakaguchi N, Sakaguchi S, Autoimmune disease as a consequence of developmental abnormity of a T cell subpopulationJ Exp Med 184: 387–396, 1996
Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T, Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory diseaseNature 359: 693–699, 1992
Powrie F, Leach MW, Mauze S, Barcomb Caddle L, Coffman RL, Phenotyically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid miceInt Immunol 5: 1461–1471, 1993
Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W, Interleukin-10-deficient mice develop chronic enterocolitis Cell 75: 263–274, 1993
Hori S, Carvalho TL, Demengeot J, CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice Eur J Immunol 32: 1282–1291, 2002
Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E, Tumor rejection by in vivo administration of anti-CD25 (Interleukin-2 receptor α) monoclonal antibodyCancer Res 59: 3128–3133, 1999
Shimizu J, Yamazaki S, Sakaguchi S, Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunityJ Immunol 163: 5211–5218, 1999
Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH, Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral bloodJ Exp Med 193: 1285–1294, 2001
Cosmi L, Liotta F, Lazzeri E, Francalanci M, Angeli R, Mazzinghi B, Santarlasci V, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S, Annunziato F, Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytesBlood102: 4107–4114, 2003
Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S, Phenotype, localization, and mechanism of suppression of CD4+CD25+ human thymocytesJ Exp Med 196: 379–387, 2002
Nakamura K, Kitani A, Strober W, Cell contact-dependent immunosupression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor βJ Exp Med 194: 629–644, 2001
Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA, Role for TGF-β in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral bloodJ Immunol 166: 7282–7289, 2001
Peng Y, Laouar Y, Li MO, Green EA, Flavell RA, TGF-β regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetesProc Natl Acad Sci USA 101: 4572–4577, 2004
Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, Roncarolo MG, Human CD25+CD4+ T suppressor cell clones produce transforming growth factor β, but not interleukin 10, and are distinct from type 1 T regulatory cellsJ Exp Med 196: 1335–1346, 2002
Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM, Conversion of peripheral CD4+CD25− naïve T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3J Exp Med 198: 1875–1886, 2003
Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF, TGF-β induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7J Immunol 172: 5149–5153, 2004
Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, Protschka M, Galle PR, Neurath MF, Blessing M, TGF-β signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cellsJ Immunol 173: 6526–6531, 2004
Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W, TGF-β1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and miceJ Immunol 172: 834–842, 2004
Woll MM, Fisher CM, Ryan GB, Gurney JM, Storrer CE, Ioannides CG, Shriver CD, Moul JW, McLeod DG, Ponniah S, Peoples GE, Direct measurement of peptide-specific CD8+ T cells using HLA-A2:Ig dimer for monitoring the in vivo immune response to a HER2/neu vaccine in breast and prostate cancer patientsJ Clin Immunol 24: 449–461, 2004
Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA, CD4+CD25high regulatory cells in human peripheral bloodJ Immunol 167:1245–1253, 2001
Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F, Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinomaCancer Res 65: 2457–2464, 2005
Cao D, Malmström V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C, Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritisEur J Immunol 33: 215–223, 2003
Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH, Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancerCancer Res 61: 4766–4772, 2001
Peoples GE, Gurney JM, Ryan GB, Hueman MT, Woll MM, Ryan GB, Storrer CE, Fisher C, Shriver CD, Ioannides CG, Ponniah S. Clinical Trial Results of a HER2/neu (E75) Vaccine to Prevent Recurrence in High-Risk Breast Cancer Patients. J Clin Oncol 23: 7536–7545
Ullenhag GJ, Frödin JE, Jeddi-Tehrani M, Strigård K, Eriksson E, Samanci A, Choudhury A, Nilsson B, Rossmann ED, Mosolits S, Mellstedt H, Durable carcinoembryonic antigen (CEA)-specific humoral and cellular immune responses in colorectal carcinoma patients vaccinated with recombinant CEA and granulocyte/macrophage colony-stimulating factorClin Cancer Res 10: 3273–3281, 2004
Slingluff CL Jr, Petroni GR, Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Hibbitts S, Teates D, Neese PY, Grosh WW, Chianese-Bullock KA, Woodson EMH, Wiernasz CJ, Merrill P, Gibson J, Ross M, Engelhard VH, Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cellsJ Clin Oncol 21: 4016–4026, 2003
Soiffer R, Hodi FS, Haluska F, Jung K, Gillessen S, Singer S, Tanabe K, Duda R, Mentzer S, Jaklitsch M, Bueno R, Clift S, Hardy S, Neuberg D, Mulligan R, Webb I, Mihm M, Dranoff G, Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanomaJ Clin Oncol 21: 3343–3350, 2003
Weber J, Sondak VK, Scotland R, Phillip R, Wang F, Rubio V, Stuge TB, Groshen SG, Gee C, Jeffery GG, Sian S, Lee PP, Granulocyte-macrophage-colony-stimulating factor added to a multipeptide vaccine for resected stage II melanoma Cancer 97: 186–200, 2003
Disis ML, Gooley TA, Rinn K, Davies D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K, Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccinesJ Clin Oncol 20: 2624–2632, 2002
Murray JL, Przepiorka D, Ioannides CG, Clinical trials of HER-2/neu-specific vaccinesSemin Oncol 27Suppl 11: 71–75, 2000
Zaks TZ, Rosenberg SA, Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumorsCancer Res 58: 4902–4908, 1998
Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diaz de Cerio A, Melero I, Prieto J, Borrás-Cuesta F, Lasarte JJ, CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-γ-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccinationJ Immunol 171: 5931–5939, 2003
Piccirillo CA, Shevach EM, Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cellsJ Immunol 167: 1137–1140, 2001
Steitz J, Brück J, Lenz J, Knop J, Tüting T, Depletion of CD25+CD4+ T cells and treatment with tyrosinase-related protein 2-transduced dendritic cells enhance the interferon α-induced, CD8+ T-cell-dependent immune defense of B16 melanomaCancer Res 61: 8643–8646, 2001
Golgher D, Jones E, Powrie F, Elliott T, Gallimore A, Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigensEur J Immunol 32: 3267–3275, 2002
Casares N, Lasarte JJ, López-Diaz de Cerio A, Sarobe P, Ruiz M, Melero I, Prieto J, Borrás-Cuesta F, Immunization with a tumor-associated CTL epitope plus a tumor-related or unrelated Th1 helper peptide elicits protective CTL immunityEur J Immunol 31: 1780–1789, 2001
North RJ, Down-regulation of the antitumor immune responseAdv Cancer Res 45:1–43, 1985
Sutmuller RPM, van Duivenvoorde LM, van Elsas A, Schumacher TNM, Wildenberg ME, Allison JP, Toes REM, Offringa R, Melief CJM, Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responsesJ Exp Med 194: 823–832, 2001
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W, Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survivalNat Med10: 942–949, 2004
Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH, Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferationJ Immunol 168: 4272–4276, 2002
Ichihara F, Kono K, Takahashi A, Kawaida H, Hidemitsu S, Fujii H, Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancersClin Cancer Res 9: 4404–4408, 2003
Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC, Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinomaJ Immunol 169: 2756–2761, 2002
Peoples GE, Goedegebuure PS, Smith R, Linehan DC, Yoshino I, Eberlein TJ, Breast and ovarian cancer-specific cytotoxic lymphocytes recognize the same HER2/neu-derived peptideProc Natl Acad Sci USA 92: 432–436, 1995
Fisk B, Blevins TL, Wharton JT, Ioannides CG, Identification of an immunodominant peptide of the HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte linesJ Exp Med 181: 2109–2117, 1995
Peiper M, Goedegebuure PS, Izbicki JR, Eberlein TJ, Pancreatic cancer associated ascites-derived CTL recognize a nine-amino-acid peptide GP2 derived from HER2/neuAnticancer Res 19: 2471–2476, 1999
Antony PA, Restifo NP, Do CD4+CD25+ immunoregulatory T cells hinder immunotherapy? J Immunother 25: 202–206, 2002
Acknowledgements
This work was supported by funds from the Department of Defense to the Henry M. Jackson Foundation for the Advancement of Military Medicine (Rockville, MD) for the Clinical Breast Care Project. We thank Ms. Diane Papay and Ms. Stacy O’Neill of the Clinical Breast Care Project who provided excellent patient care and administration of the clinical trial. We also thank the staff of the Clinical Breast Care Project Immunology and Research Center for their clinical and administrative assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of the Army or the Department of Defense
Rights and permissions
About this article
Cite this article
Hueman, M.T., Stojadinovic, A., Storrer, C.E. et al. Levels of circulating regulatory CD4+CD25+ T cells are decreased in breast cancer patients after vaccination with a HER2/neu peptide (E75) and GM-CSF vaccine★ . Breast Cancer Res Treat 98, 17–29 (2006). https://doi.org/10.1007/s10549-005-9108-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-005-9108-5