Abstract
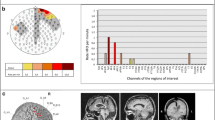
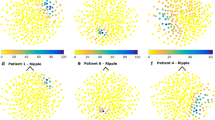
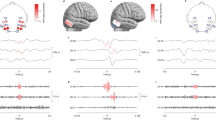
High frequency oscillations (HFOs) are emerging as biomarkers of epileptogenicity. They have been shown to originate from small brain regions. Surprisingly, spontaneous HFOs can be recorded from the scalp. To understand how is it possible to observe these small events on the scalp, one avenue is the analysis of the cortical correlates at the time of scalp HFOs. Using simultaneous scalp and intracranial recordings of 11 patients, we studied the spatial distribution of scalp events on the cortical surface. For typical interictal epileptiform discharges the subdural distributions were, as expected, spatially extended. On the contrary, for scalp HFOs the subdural maps corresponded to focal sources, consisting of one or a few small spatial extent activations. These topographies suggest that small cortical areas generated the HFOs seen on the scalp. Similar scalp distributions corresponded to distinct distributions on a standard 1 cm subdural grid and averaging similar scalp HFOs resulted in focal subdural maps. The assumption that a subdural grid “sees” everything that contributes to the potential of nearby scalp contacts was not valid for HFOs. The results suggest that these small extent events are spatially undersampled with standard scalp and grid inter-electrode distances. High-density scalp electrode distributions seem necessary to obtain a solid sampling of HFOs on the scalp. A better understanding of the influence of spatial sampling on the observation of high frequency brain activity on the scalp is important for their clinical use as biomarkers of epilepsy.










Similar content being viewed by others
References
Alarcon G, Guy CN, Binnie CD, Walker SR, Elwes RD, Polkey CE (1994) Intracerebral propagation of interictal activity in partial epilepsy: implications for source localisation. J Neurol Neurosurg Psychiatry 57(4):435–449
Andrade-Valença LP, Dubeau F, Mari F, Zelmann R, Gotman J (2011) Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology 77(6):524–531. doi:10.1212/WNL.0b013e318228bee2
Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J (2009) Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia 50(4):617–628
Ball T, Demandt E, Mutschler I, Neitzel E, Mehring C, Vogt K, Aertsen A, Schulze-Bonhage A (2008) Movement related activity in the high gamma range of the human EEG. Neuroimage 41(2):302–310
Bénar CG, Chauviere L, Bartolomei F, Wendling F (2010) Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on “false” ripples. Clin Neurophysiol 121(3):301–310
Bénar CG, Gotman J (2002) Modeling of post-surgical brain and skull defects in the EEG inverse problem with the boundary element method. Clin Neurophysiol 113(1):48–56
Boonyapisit K, Najm I, Klem G, Ying Z, Burrier C, LaPresto E, Nair D, Bingaman W, Prayson R, Luders H (2003) Epileptogenicity of focal malformations due to abnormal cortical development: direct electrocorticographic-histopathologic correlations. Epilepsia 44(1):69–76
Bragin A, Mody I, Wilson CL, Engel J Jr (2002) Local generation of fast ripples in epileptic brain. J Neurosci 22(5):2012–2021
Brodbeck V, Spinelli L, Lascano AM, Wissmeier M, Vargas MI, Vulliemoz S, Pollo C, Schaller K, Michel CM, Seeck M (2011) Electroencephalographic source imaging: a prospective study of 152 operated epileptic patients. Brain 134(Pt 10):2887–2897. doi:10.1093/brain/awr243
Buzsáki G, Draguhn A (2004) Neuronal oscillations in cortical networks. Science 304(5679):1926–1929. doi:10.1126/science.1099745
Chrobak JJ, Buzsaki G (1996) High-frequency oscillations in the output networks of the hippocampal–entorhinal axis of the freely behaving rat. J Neurosci 16(9):3056–3066
Cooper R, Winter AL, Crow HJ, Walter WG (1965) Comparison of subcortical, cortical and scalp activity using chronically indwelling electrodes in man. Electroencephalogr Clin Neurophysiol 18:217–228
Cosandier-Rimele D, Merlet I, Badier JM, Chauvel P, Wendling F (2008) The neuronal sources of EEG: modeling of simultaneous scalp and intracerebral recordings in epilepsy. Neuroimage 42(1):135–146. doi:10.1016/j.neuroimage.2008.04.185
Crepon B, Navarro V, Hasboun D, Clemenceau S, Martinerie J, Baulac M, Adam C, Le Van Quyen M (2010) Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain 133(Pt 1):33–45. doi:10.1093/brain/awp277
Curio G (2000) Linking 600-Hz “spikelike” EEG/MEG wavelets (“sigma-bursts”) to cellular substrates: concepts and caveats. J Clin Neurophysiol 17(4):377–396
Duun-Henriksen J, Kjaer TW, Madsen RE, Jespersen B, Duun-Henriksen AK, Remvig LS, Thomsen CE, Sorensen HB (2013) Subdural to subgaleal EEG signal transmission: the role of distance, leakage and insulating affectors. Clin Neurophysiol. doi:10.1016/j.clinph.2013.02.112
Freeman WJ, Rogers LJ, Holmes MD, Silbergeld DL (2000) Spatial spectral analysis of human electrocorticograms including the alpha and gamma bands. J Neurosci Methods 95(2):111–121
Gambardella A, Palmini A, Andermann F, Dubeau F, Da Costa JC, Quesney LF, Andermann E, Olivier A (1996) Usefulness of focal rhythmic discharges on scalp EEG of patients with focal cortical dysplasia and intractable epilepsy. Electroencephalogr Clin Neurophysiol 98(4):243–249
Gloor P (1985) Neuronal generators and the problem of localization in electroencephalography: application of volume conductor theory to electroencephalography. J Clin Neurophysiol 2(4):327–354
Goncalves SI, de Munck JC, Verbunt JP, Bijma F, Heethaar RM, Lopes da Silva FH (2003) In vivo measurement of the brain and skull resistivities using an EIT-based method and realistic models for the head. IEEE Trans Biomed Eng 50(6):754–767
Gramfort A, Papadopoulo T, Olivi E, Clerc M (2010) OpenMEEG: opensource software for quasistatic bioelectromagnetics. Biomed Eng Online 9:45. doi:10.1186/1475-925X-9-45
Grenier F, Timofeev I, Steriade M (2001) Focal synchronization of ripples (80–200 Hz) in neocortex and their neuronal correlates. J Neurophysiol 86(4):1884–1898
Hashiguchi K, Morioka T, Yoshida F, Miyagi Y, Nagata S, Sakata A, Sasaki T (2007) Correlation between scalp-recorded electroencephalographic and electrocorticographic activities during ictal period. Seizure 16(3):238–247. doi:10.1016/j.seizure.2006.12.010
Heasman BC, Valentin A, Alarcon G, Garcia Seoane JJ, Binnie CD, Guy CN (2002) A hole in the skull distorts substantially the distribution of extracranial electrical fields in an in vitro model. J Clin Neurophysiol 19(2):163–171
Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC (1998) Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22(2):324–333
Jacobs J, Levan P, Chatillon CE, Olivier A, Dubeau F, Gotman J (2009) High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain 132(Pt 4):1022–1037
Jacobs J, Staba R, Asano E, Otsubo H, Wu JY, Zijlmans M, Mohamed I, Kahane P, Dubeau F, Navarro V, Gotman J (2012) High-frequency oscillations (HFOs) in clinical epilepsy. Prog Neurobiol. doi:10.1016/j.pneurobio.2012.03.001
Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J (2006) High-frequency oscillations during human focal seizures. Brain 129(Pt 6):1593–1608
Kobayashi K, Oka M, Akiyama T, Inoue T, Abiru K, Ogino T, Yoshinaga H, Ohtsuka Y, Oka E (2004) Very fast rhythmic activity on scalp EEG associated with epileptic spasms. Epilepsia 45(5):488–496. doi:10.1111/j.0013-9580.2004.45703.x
Kobayashi K, Watanabe Y, Inoue T, Oka M, Yoshinaga H, Ohtsuka Y (2010) Scalp-recorded high-frequency oscillations in childhood sleep-induced electrical status epilepticus. Epilepsia 51(10):2190–2194. doi:10.1111/j.1528-1167.2010.02565.x
Kobayashi K, Yoshinaga H, Ohtsuka Y, Gotman J (2005) Dipole modeling of epileptic spikes can be accurate or misleading. Epilepsia 46(3):397–408. doi:10.1111/j.0013-9580.2005.31404.x
Kybic J, Clerc M, Abboud T, Faugeras O, Keriven R, Papadopoulo T (2005) A common formalism for the integral formulations of the forward EEG problem. IEEE Trans Med Imaging 24(1):12–28
Lachaux JP, Rodriguez E, Martinerie J, Varela FJ (1999) Measuring phase synchrony in brain signals. Hum Brain Mapp 8(4):194–208
Lai Y, van Drongelen W, Ding L, Hecox KE, Towle VL, Frim DM, He B (2005) Estimation of in vivo human brain-to-skull conductivity ratio from simultaneous extra- and intra-cranial electrical potential recordings. Clin Neurophysiol 116(2):456–465. doi:10.1016/j.clinph.2004.08.017
Lanfer B, Roer C, Scherg M, Rampp S, Kellinghaus C, Wolters C (2013) Influence of a silastic ECoG grid on EEG/ECoG based source analysis. Brain Topogr 26(2):212–228. doi:10.1007/s10548-012-0251-0
Lantz G, Grave De Peralta R, Spinelli L, Seeck M, Michel CM (2003) Epileptic source localization with high density EEG: how many electrodes are needed? Clin Neurophysiol 114(1):63–69
Law SK (1993) Thickness and resistivity variations over the upper surface of the human skull. Brain Topogr 6:99–109
Lu Y, Yang L, Worrell GA, He B (2012) Seizure source imaging by means of FINE spatio-temporal dipole localization and directed transfer function in partial epilepsy patients. Clin Neurophysiol 123(7):1275–1283. doi:10.1016/j.clinph.2011.11.007
Mégevand P, Spinelli L, Genetti M, Brodbeck V, Momjian S, Schaller K, Vulliemoz S, Seeck M (2013) Electric source imaging of interictal activity accurately localises the seizure onset zone. J Neurol Neurosurg Psychiatry. doi:10.1136/jnnp-2013-305515
Melani F, Zelmann R, Dubeau F, Gotman J (2013) Occurrence of scalp-fast oscillations among patients with different spiking rate and their role as epileptogenicity marker. Epilepsy Res. doi:10.1016/j.eplepsyres.2013.06.003
Michel CM, Murray MM (2012) Towards the utilization of EEG as a brain imaging tool. Neuroimage 61:371–385. doi:10.1016/j.neuroimage.2011.12.039
Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave De Peralta R (2004a) EEG source imaging. Clin Neurophysiol 10:2195–2222
Michel CM, Lantz G, Spinelli L, De Peralta RG, Landis T, Seeck M (2004b) 128-Channel EEG source imaging in epilepsy: clinical yield and localization precision. J Clin Neurophysiol 21:71–83
Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, Miller JW, Ojemann JG (2007) Spectral changes in cortical surface potentials during motor movement. J Neurosci 27(9):2424–2432. doi:10.1523/JNEUROSCI.3886-06.2007
Oishi M, Otsubo H, Kameyama S, Morota N, Masuda H, Kitayama M, Tanaka R (2002) Epileptic spikes: magnetoencephalography versus simultaneous electrocorticography. Epilepsia 43(11):1390–1395
Oostendorp TF, Delbeke J, Stegeman DF (2000) The conductivity of the human skull: results of in vivo and in vitro measurements. IEEE Trans Biomed Eng 47(11):1487–1492. doi:10.1109/TBME.2000.880100
Ramon C, Holmes MD (2013) Noninvasive epileptic seizure localization from stochastic behavior of short duration interictal high density scalp EEG data. Brain Topogr 1:106–115. doi:10.1007/s10548-011-0188-8
Ryynanen OR, Hyttinen JA, Laarne PH, Malmivuo JA (2004) Effect of electrode density and measurement noise on the spatial resolution of cortical potential distribution. IEEE Trans Biomed Eng 51(9):1547–1554. doi:10.1109/TBME.2004.828036
Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J Jr (2004) High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol 56(1):108–115
Storti SF, Boscolo Galazzo I, Del Felice A, Pizzini FB, Arcaro C, Formaggio E, Mai R, Manganotti P (2013) Combining ESI. ASL and PET for quantitative assessment of drug-resistant focal epilepsy. Neuroimage. doi:10.1016/j.neuroimage.2013.06.028
Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM (2011) Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011:879716. doi:10.1155/2011/879716
Tang C, You F, Cheng G, Gao D, Fu F, Yang G, Dong X (2008) Correlation between structure and resistivity variations of the live human skull. IEEE Trans Biomed Eng 55(9):2286–2292. doi:10.1109/TBME.2008.923919
Tao JX, Baldwin M, Hawes-Ebersole S, Ebersole JS (2007) Cortical substrates of scalp EEG epileptiform discharges. J Clin Neurophysiol 24(2):96–100
Urrestarazu E, Chander R, Dubeau F, Gotman J (2007) Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain 130(Pt 9):2354–2366
von Ellenrieder N, Andrade-Valenca LP, Dubeau F, Gotman J (2012) Automatic detection of fast oscillations (40–200 Hz) in scalp EEG recordings. Clin Neurophysiol 123(4):670–680. doi:10.1016/j.clinph.2011.07.050
Worrell GA, Jerbi K, Kobayashi K, Lina JM, Zelmann R, Le Van Quyen M (2012) Recording and analysis techniques for high-frequency oscillations. Prog Neurobiol. doi:10.1016/j.pneurobio.2012.02.006
Wu JY, Koh S, Sankar R, Mathern GW (2008) Paroxysmal fast activity: an interictal scalp EEG marker of epileptogenesis in children. Epilepsy Res 82(1):99–106. doi:10.1016/j.eplepsyres.2008.07.010
Yamazaki M, Tucker D, Terrill M, Fujimoto A, Yamamoto T (2013) Dense array EEG (dEEG) source estimation in neocortical epilepsy. Front Neurol 4:42. doi:10.3389/fneur.2013.00042
Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY (2008) Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron 58(3):429–441. doi:10.1016/j.neuron.2008.03.027
Zelmann R, Mari F, Jacobs J, Zijlmans M, Chander R, Gotman J (2010) Automatic detector of high frequency oscillations for human recordings with macroelectrodes. Conf Proc IEEE Eng Med Biol Soc 2010:2329–2333. doi:10.1109/IEMBS.2010.5627464
Zelmann R, Mari F, Jacobs J, Zijlmans M, Dubeau F, Gotman J (2012) A comparison between detectors of high frequency oscillations. Clin Neurophysiol 123(1):106–116. doi:10.1016/j.clinph.2011.06.006
Zijlmans M, Jiruska P, Zelmann R, Leijten FS, Jefferys JG, Gotman J (2012) High-frequency oscillations as a new biomarker in epilepsy. Ann Neurol 71(2):169–178. doi:10.1002/ana.22548
Acknowledgments
We thank Dr. Federico Melani for his help in the identification of interictal epileptiform discharges. We thank Francois Tadel and Dr. Sylvain Baillet for their suggestions on how to implement brainstorm for our simulations. We are grateful to Dr. Matthias Dümpelmann for his help in obtaining the data. This project was founded in part by DAAD short-term scholarship, the Savoy Foundation, and grant MOP-102,710 from the Canadian Institutes of Health Research. JJ was supported by the German Research Foundation (grant JA 1,725/2-1).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Linear Model
There is a linear relation between subdural contacts and scalp, since tissue in between (skull, scalp, dura) is purely resistive at the frequencies of interest. Thus, it is possible to create a linear model of the form
or in matrix form (ignoring the error term)
where mScalp is an M×N matrix containing the concatenated scalp EEG signal (\(sScalp_{Chi}^{HFOi} )\); mIEEG is a K×N matrix containing the concatenated intracranial EEG signal at the time of the scalp events (\(sIEEG_{ChM}^{HFO1} )\); and GM×K is the forward model to estimate. M is the number of scalp channels; K is the number of subdural channels; and N is the number of scalp events.
Assuming that the noise is Gaussian the matrix GM×K can be obtained from the training set by
where train are the training events; ′ is the transpose operator; + is the inverse of the matrix (pseudo inverse since these are non-square matrices). GM×K is estimated for all but one event at each run. We estimated the matrix GM×K using the backslash operator in Matlab which provides the least square solution. At each run the estimated mScalptest event was obtained as
This procedure was repeated for all events in the leave one out schema.
Rights and permissions
About this article
Cite this article
Zelmann, R., Lina, J.M., Schulze-Bonhage, A. et al. Scalp EEG is not a Blur: It Can See High Frequency Oscillations Although Their Generators are Small. Brain Topogr 27, 683–704 (2014). https://doi.org/10.1007/s10548-013-0321-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-013-0321-y