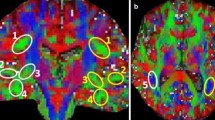
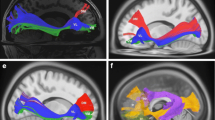
Abstract
Traditional models of the human language circuitry encompass three cortical areas, Broca’s, Geschwind’s and Wernicke’s, and their connectivity through white matter fascicles. The neural connectivity deep to these cortical areas remains poorly understood, as does the macroscopic functional organization of the cortico-subcortical language circuitry. In an effort to expand current knowledge, we combined functional MRI (fMRI) and diffusion tensor imaging to explore subject-specific structural and functional macroscopic connectivity, focusing on Broca’s area. Fascicles were studied using diffusion tensor imaging fiber tracking seeded from volumes placed manually within the white matter. White matter fascicles and fMRI-derived clusters (antonym-generation task) of positive and negative blood-oxygen-level-dependent (BOLD) signal were co-registered with 3-D renderings of the brain in 12 healthy subjects. Fascicles connecting BOLD-derived clusters were analyzed within specific cortical areas: Broca’s, with the pars triangularis, the pars opercularis, and the pars orbitaris; Geschwind’s and Wernicke’s; the premotor cortex, the dorsal supplementary motor area, the middle temporal gyrus, the dorsal prefrontal cortex and the frontopolar region. We found a functional connectome divisible into three systems—anterior, superior and inferior—around the insula, more complex than previously thought, particularly with respect to a new extended Broca’s area. The extended Broca’s area involves two new fascicles: the operculo-premotor fascicle comprised of well-organized U-shaped fibers that connect the pars opercularis with the premotor region; and (2) the triangulo-orbitaris system comprised of intermingled U-shaped fibers that connect the pars triangularis with the pars orbitaris. The findings enhance our understanding of language function.





Similar content being viewed by others
References
Anwander A et al (2007) Connectivity-based parcellation of broca’s area. Cereb Cortex (New York, 1991) 17(4):816–825
Basser Peter J, Jones DK (2002) Diffusion-tensor MRI: theory, experimental design and data analysis-a technical review. NMR Biomed 15(7–8):456–467
Basser PJ, Roth BJ (2000) New currents in electrical stimulation of excitable tissues. Annu Rev Biomed Eng 2:377–397
Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66(1):259–267
Basser PJ et al (2000) In vivo fiber tractography using DT-MRI data. Magn Reson Med 44(4):625–632
Bechtereva NP, Abdullaev YG, Medvedev SV (1991) Neuronal activity in frontal speech area 44 of the human cerebral cortex during word recognition. Neurosci Lett 124(1):61–64
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc 57:289–300
Benson D, Ardilla A (1995) Conduction aphasia: a syndrome of language network disruption. In: Kirshner HS (ed) Handbook of neurological speech and language disorders. Dekker, New York, pp 149–164
Boatman D et al (2000) Transcortical sensory aphasia: revisited and revised. Brain 123(8):1634–1642
Broca P (1861) Perte de la parole, ramollissement chronique et destruction partielle du lobe antérieur gauche du cerveau. Bull de la Société d’Anthroplogie de Paris 2:235–238
Catani M, Jones DK, ffytche DH (2005) Perisylvian language networks of the human brain. Ann Neurol 57(1):8–16
Catani M et al (2007) Symmetries in human brain language pathways correlate with verbal recall. Proc Nat Acad Sci USA 104(43):17163–17168
Davis C et al (2008) Speech and language functions that require a functioning broca’s area. Brain Lang 105(1):50–58
De Carli D et al (2007) Identification of activated regions during a language task. Magn Reson Imaging 25(6):933–938
Dejerine J (1901) Anatomie des centres nerveux (Tomes 1 and 2). Rueff Cie, Paris
Desmond JE et al (1995) Functional MRI measurement of language lateralization in Wada-tested patients. Brain 118(6):1411–1419
Dronkers NF et al (2007) Paul Broca’s historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain 130(Pt 5):1432–1441
Duffau H et al (2002) Intraoperative mapping of the subcortical language pathways using direct stimulations. an anatomo-functional study. Brain 125(Pt 1):199–214
Duffau H et al (2005) New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain 128(Pt 4):797–810
Duvernoy H et al (1992) Le cerveau humain: Surfaces, coupes sériées tridimentionelles et IRM. Springer, Paris
Fink M et al (2009) Lateralization of the serotonin-1A receptor distribution in language areas revealed by PET. NeuroImag 45(2):598–605
Frey S et al (2008) Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci 28(45):11435–11444
Friederici Angela D (2009) Pathways to language: fiber tracts in the human brain. Trends Cogn Sci 13(4):175–181
Friston K et al (1995) Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2:189–210
Geschwind N (1970) The organization of language and the brain. Science (New York, NY) 170(961):940–944
Glasser MF, Rilling JK (2008) DTI tractography of the human brain’s language pathways. Cereb Cortex (New York, 1991) 18(11):2471–2482
Green AE et al (2006) Frontopolar cortex mediates abstract integration in analogy. Brain Res 1096(1):125–137
Hatsopoulos NG, Donoghue JP (2009) The science of neural interface systems. Annu Rev Neurosci 32:249–266
Indefrey P, Levelt WJM (2004) The spatial and temporal signatures of word production components. Cognition 92(1–2):101–144
Jaermann T et al (2008) Preliminary experience with visualization of intracortical fibers by focused high-resolution diffusion tensor imaging. Am J Neuroradiol 29(1):146–150
Keller SS et al (2009) Broca’s area: nomenclature, anatomy, typology and asymmetry. Brain Lang 109(1):29–48
Kirshner H (1995) Classical aphasia syndromes. In: Kirshner HS (ed) Handbook of neurological speech and language disorders. Dekker, New York, pp 57–89
Klingler J (1935) Erleichterung des makroskopischen praeparation des gehirns durch den gefrierprozess. Schweiz Arch Neurol Psychiatr 36:247–256
Le Bihan D (2003) Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 4(6):469–480
Lee HW et al (2009) Reorganisation of cortical motor and language distribution in human brain. J Neurol Neurosurg Psychiatry 80(3):285–290
Leow AD et al (2009) The tensor distribution function. Magn Reson Med 61(1):205–214
Logothetis NK (2002) The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci 357(1424):1003–1037
Makris N et al (2005) Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo DT-MRI study. Cereb Cortex (New York, 1991) 15(6):854–869
Mandonnet E et al (2007) Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain 130(Pt 3):623–629
Manola L et al (2007) Anodal vs cathodal stimulation of motor cortex: a modeling study. Clin Neurophysiol 118(2):464–474
McKiernan KA et al (2003) A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci 15(3):394–408
Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51(5):527–539
Mozolic JL et al (2008) Cross-modal deactivations during modality-specific selective attention. BMC Neurology 8:35
Nieuwenhuys R, Voogd J, Huijzen C (1979) The human central nervous system: a synopsis and atlas. Springer, Berlin
Nowak MA, Komarova NL, Niyogi P (2002) Computational and evolutionary aspects of language. Nature 417(6889):611–617
Ojemann GA (2003) The neurobiology of language and verbal memory: observations from awake neurosurgery. Intern J Psychophysiol 48(2):141–146
Ojemann G et al (1989) Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg 71(3):316–326
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Parker GJM et al (2005) Lateralization of ventral and dorsal auditory-language pathways in the human brain. NeuroImag 24(3):656–666
Petrides M, Pandya DN (2009) Distinct parietal and temporal pathways to the homologues of Broca’s area in the monkey. PLoS Biol 7(8):e1000170
Pulvermüller F (2005) Brain mechanisms linking language and action. Nat Rev Neurosci 6(7):576–582
Quigg M, Fountain NB (1999) Conduction aphasia elicited by stimulation of the left posterior superior temporal gyrus. J Neurol Neurosurg Psychiatry 66(3):393–396
Riley H (1953) An atlas of the basal ganglia, brain stem and spinal cord. Williams and Wilkins, Baltimore
Rizzolatti G, Craighero L (2004) The mirror-neuron system. Annu Rev Neurosci 27:169–192
Sandrini M, Rusconi E (2009) A brain for numbers. Cortex 45(7):796–803
Saur D et al (2008) Ventral and dorsal pathways for language. Proc Nat Acad Sci USA 105(46):18035–18040
Schmahmann J, Pandya D (2006) Fiber pathways of the brain. Oxford University Press, New York
Schmahmann JD et al (2008) Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci 1142:266–309
Simmons-Mackie N (1997) Conduction aphasia. In: LaPointe LL (ed) Aphasia and related neurogenic language disorders. Thiema, New York, pp 63–90
Sotero RC, Trujillo-Barreto NJ (2007) Modelling the role of excitatory and inhibitory neuronal activity in the generation of the BOLD signal. NeuroImag 35(1):149–165
Sporns O, Tononi G, Kötter R (2005) The human connectome: a structural description of the human brain. PLoS Comput Biol 1(4):e42
Tie Y et al (2009) Comparison of blocked and event-related fMRI designs for pre-surgical language mapping. NeuroImag 47(Suppl 2):T107–T115
Tsapkini K, Vivas AB, Triarhou LC (2008) « Does Broca » s area exist?’ Christofredo Jakob’s 1906 response to Pierre Marie’s holistic stance. Brain Lang 105(3):211–219
Tuch DS (2004) Q-ball imaging. Magn Reson Med 52(6):1358–1372
Vernooij MW et al (2007) Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. NeuroImag 35(3):1064–1076
Acknowledgments
We thank Wentao Wu, Isaiah Norton, Wendy Plesniak, and Nicole Aucoin for their technical support. We thank Ann Adams for her editorial advice. We are grateful to the community and major sponsors and contributors to Slicer (http://www.slicer.org/pages/Acknowledgments) who permitted us to use the most recent technologies of real-time fiber tracking. This work is part of the National Alliance for Medical Image Computing (NA-MIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149. Information on the National Centers for Biomedical Computing can be obtained from http://nihroadmap.nih.gov/bioinformatics. This work was also supported in part by NIH grants U41-RR019703, P41-RR13218, and P01 CA067165 and by the ITMO Health Technologies (Institut Thématique Multi-Organismes Technologies pour la Santé).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lemaire, JJ., Golby, A., Wells, W.M. et al. Extended Broca’s Area in the Functional Connectome of Language in Adults: Combined Cortical and Subcortical Single-Subject Analysis Using fMRI and DTI Tractography. Brain Topogr 26, 428–441 (2013). https://doi.org/10.1007/s10548-012-0257-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-012-0257-7