Abstract
Background
Lysosomal storage diseases (LSDs) are inborn errors of metabolism resulting from 50 different inherited disorders. The increasing availability of treatments and the importance of early intervention have stimulated newborn screening (NBS) to diagnose LSDs and permit early intervention to prevent irreversible impairment or severe disability. We present our experience screening newborns in North East Italy to identify neonates with Mucopolysaccharidosis type I (MPS I) and Pompe, Fabry, and Gaucher diseases.
Methods
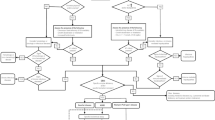
Activities of acid β-glucocerebrosidase (ABG; Gaucher), acid α-glucosidase (GAA; Pompe), acid α-galactosidase (GLA; Fabry), and acid α-L-iduronidase (IDUA; MPS-I) in dried blood spots (DBS) from all newborns during a 17-month period were determined by multiplexed tandem mass spectrometry (MS/MS) using the NeoLSD® assay system. Enzymatic activity cutoff values were determined from 3500 anonymous newborn DBS. In the screening study, samples were retested if the value was below cutoff and a second spot was requested, with referral for confirmatory testing and medical evaluation if a low value was obtained.
Results
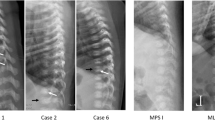
From September 2015 to January 2017, 44,411 newborns were screened for the four LSDs. We recalled 40 neonates (0.09%) for collection of a second DBS. Low activity was confirmed in 20, who had confirmatory testing. Ten of 20 had pathogenic mutations: two Pompe, two Gaucher, five Fabry, and one MPS-I. The incidences of Pompe and Gaucher diseases were similar (1/22,205), with Fabry disease the most frequent (1/8882) and MPS-I the rarest (1/44411). The combined incidence of the four disorders was 1/4411 births.
Conclusions
Simultaneously determining multiple enzyme activities by MS/MS, with a focus on specific biochemical markers, successfully detected newborns with LSDs. The high incidence of these disorders supports this screening program.



Similar content being viewed by others
Abbreviations
- ABG:
-
Acid β-glucocerebrosidase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CPK:
-
Creatine phosphokinase
- CRIM:
-
Cross-reactive immunological material
- DBS:
-
Dried blood spot
- EA:
-
Ethyl acetate
- ECG:
-
Electrocardiogram
- ENBS:
-
Expanded newborn screening
- ERT:
-
Enzyme replacement therapy
- GAA:
-
Acid α-glucosidase
- GlcSPh:
-
Glucosylsphingosine
- IDUA:
-
Acid α-L-iduronidase
- LSD:
-
Lysosomal storage disease
- LysoGb3:
-
Globotriaosylsphingosine
- MeOH:
-
Methanol
- MOM:
-
Multiple of the median
- MPS I:
-
Mucopolysaccharidosis type I
- NBS:
-
Newborn screening
- MRM:
-
Multiple reaction monitoring
- PPV:
-
Positive predictive value
References
Al-Sannaa NA, Bay L, Barbouth DS et al (2015) Early treatment with laronidase improves clinical outcomes in patients with attenuated MPS I: a retrospective case series analysis of nine sibships. Orphanet J Rare Dis 10:131
Burton BK, Charrow J, Hoganson GE et al (2017) Newborn screening for Lysosomal storage disorders in Illinois: the initial 15-month experience. J Pediatr. https://doi.org/10.1016/j.jpeds.2017.06.048
Chien YH, Lee NC, Thurberg BL et al (2009) Pompe disease in infants: improving the prognosis by newborn screening and early treatment. Pediatrics 124:e1116–1125
Clarke LA, Atherton AM, Burton BK et al (2017) Mucopolysaccharidosis type I newborn screening: best practices for diagnosis and management. J Pediatr 182:363–370
Dionisi-Vici C, Rizzo C, Burlina AB et al (2002) Inborn errors of metabolism in the Italian pediatric population: a national retrospective survey. J Pediatr 140:321–327
Elliott S, Buroker N, Cournoyer JJ et al (2016) Pilot study of newborn screening for six lysosomal storage diseases using Tandem Mass Spectrometry. Mol Genet Metab 118:304–309
Ferreira S, Ortiz A, Germain DP et al (2015) The alpha-galactosidase A p.Arg118Cys variant does not cause a Fabry disease phenotype: data from individual patients and family studies. Mol Genet Metab 114:248–258
Fletcher JM (2006) Screening for lysosomal storage disorders–a clinical perspective. J Inherit Metab Dis 29:405–408
Fuller M, Meikle PJ, Hopwood JJ (2006) Epidemiology of lysosomal storage diseases: an overview. In: Mehta A, Beck M, Sunder-Plassmann G (eds) Fabry disease: perspectives from 5 years of FOS. Oxford PharmaGenesis, Oxford
Gelb MH, Scott CR, Turecek F (2015) Newborn screening for lysosomal storage diseases. Clin Chem 61:335–346
Germain DP, Brand E, Cecchi F et al (2017) The phenotypic characteristics of the p.N215S Fabry disease genotype in male and female patients: a multi-center Fabry Registry study. Mol Genet Metab 12:S51–S52
Hoffmann GF, Lindner M, Loeber JG (2014) 50 years of newborn screening. J Inherit Metab Dis 37:163–164
Hopkins PV, Campbell C, Klug T, Rogers S, Raburn-Miller J, Kiesling J (2015) Lysosomal storage disorder screening implementation: findings from the first six months of full population pilot testing in Missouri. J Pediatr 166:172–177
Hsu TR, Hung SC, Chang FP et al (2016) Later onset Fabry disease, cardiac damage progress in silence: experience with a highly prevalent mutation. J Am Coll Cardiol 68:2554–2563
Kemper AR, Green NS, Calonge N et al (2014) Decision-making process for conditions nominated to the recommended uniform screening panel: statement of the US Department of Health and Human Services Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Genet Med 16:183–187
Klein AD, Futerman AH (2013) Lysosomal storage disorders: old diseases, present and future challenges. Pediatr Endocrinol Rev 11(Suppl 1):59–63
Kubaski F, Mason RW, Nakatomi A et al (2017) Newborn screening for mucopolysaccharidoses: a pilot study of measurement of glycosaminoglycans by tandem mass spectrometry. J Inherit Metab Dis 40:151–158
Kumar AB, Masi S, Ghomashchi F et al (2015) Tandem mass spectrometry has a larger analytical range than fluorescence assays of lysosomal enzymes: Application to newborn screening and diagnosis of mucopolysaccharidoses types II, IVA, and VI. Clin Chem 61:1363–1371
Landau YE, Wiasbren SE, Chan LM, Levy HL (2017) Long-term outcome of expanded newborn screening at boston children’s hospital: benefits and challenges in fefinig true diseases. J Inherit Metab Dis 40:209–218
Lenders M, Weidemann F, Kurschat C et al (2016) Alpha-galactosidase A p.A143T, a non-Fabry disease-causing variant. Orphanet J Rare Dis 11:54
Li Y, Scott CR, Chamoles NA et al (2004) Direct Multiplex Assay of Lysosomal Enzymes in Dried Blood Spots for Newborn Screening. Clin Chem 50:1785–1796
Liao H-C, Chan MJ, Yang CF, et al (2017) Mass spectrometry but not fluorimetry distinguishes affected and pseudodeficiency patients in newborn screening for Pompe disease. Clin Chem 63:1271-1277
Liao H-C, Chiang CC, Niu DM et al (2014) Detecting multiple lysosomal storage diseases by tandem mass spectrometry–a national newborn screening program in Taiwan. Clin Chim Acta 431:80–86
Liao H-C, Huang Y-H, Chen Y-J et al (2013) Plasma globotriaosylsphingosine (lysoGb3) could be a biomarker for Fabry disease with a Chinese hotspot late-onset mutation (IVS4 + 919G>A). Clin Chim Acta 426:114–120
Lisi EC, McCandless SE (2016) Newborn screening for lysosomal storage disorders: views of genetic healthcare providers. J Genet Couns 25:373–384
Marsden D, Levy H (2010) Newborn screening of lysosomal storage disorders. Clin Chem 56:1071–1079
Mashima R, Sakai E, Kosuga M, Okuyama T (2016) Levels of enzyme activities in six lysosomal storage diseases in Japanese neonates determined by liquid chromatography-tandem mass spectrometry. Mol Genet Metab Rep 9:6–11
Matern D, Gavrilov D, Oglesbee D, Raymond K, Rinaldo P, Tortorelli S (2015) Newborn screening for lysosomal storage disorders. Semin Perinatol 39:206–216
Mechtler TP, Stary S, Metz TF et al (2012) Neonatal screening for lysosomal storage disorders: feasibility and incidence from a nationwide study in Austria. Lancet 379:335–341
Meikle PJ, Hopwood JJ, Clague AE, Carey WF (1999) Prevalence of lysosomal storage disorders. JAMA 281:249–254
Mistry PK (2012) Genetics and diagnosis of Gaucher disease. Clin Adv Hematol Oncol 10:7–9
Murugesan V, Chuang WL, Liu J et al (2016) Glucosylsphingosine is a key biomarker of Gaucher disease. Am J Hematol 91:1082–1089
Navarrete-Martinez JI, Limon-Rojas AE, Gaytan-Garcia MJ et al (2017) Newborn screening for six lysosomal storage disorders in a cohort of Mexican patients: three-year findings from a screening program in a closed Mexican health system. Mol Genet Metab 121:16–21
Orsini JJ, Morrissey MA, Slavin LN et al (2009) Implementation of newborn screening for Krabbe disease: population study and cutoff determination. Clin Biochem 42:877–884
Paciotti S, Persichetti E, Pagliardini S et al (2012) First pilot newborn screening for four lysosomal storage diseases in an Italian region: identification and analysis of a putative causative mutation in the GBA gene. Clin Chim Acta 413:1827–1831
Pan X, Ouyang Y, Wang Z et al (2016) Genotype: a crucial but not unique factor affecting the clinical phenotypes in Fabry disease. PLoS One 11:e0161330
Peake RW, Marsden DL, Bodamer OA, Gelb MH, Millington DS, Wijburg F (2016) Newborn screening for lysosomal storage disorders: Quo Vadis? Clin Chem 62:1430–1438
Polo G, Burlina AP, Kolamunnage TB et al (2017) Diagnosis of sphingolipidoses: a new simultaneous measurement of lysosphingolipids by LC-MS/MS. Clin Chem Lab Med 55:403–414
Schultz ML, Tecedor L, Chang M, Davidson BL (2011) Clarifying lysosomal storage diseases. Trends Neurosci 34:401–410
Scott CR, Elliott S, Buroker N et al (2013) Identification of infants at risk for developing Fabry, Pompe, or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J Pediatr 163:498–503
Serebrinsky G, Calvo M, Fernandez S et al (2015) Late onset variants in Fabry disease: Results in high risk population screenings in Argentina. Mol Genet Metab Rep 4:19–24
Singh S (2016) Status of screening for recommended disorders in the US Conference Status of screening for recommended disorders in the US, Jefferson City, MO. Accessed 28 Feb 2017
Spada M, Pagliardini S, Yasuda M et al (2006) High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet 79:31–40
Tebani A, Zanoutene-Cheriet L, Adjtoutah Z, et al (2016) Clinical and molecular characterization of patients with mucopolysaccharidosis type I in an Algerian series. Int J Mol Sci. https://doi.org/10.3390/ijms17050743
Villoria IG, Pajares S, Lopez MR, Marin JL, Ribes A (2016) Neonatal screening for inherited metabolic diseases in 2016. Semin Pediatr Neurol 23:257–272
Wilcken B, Wiley V, Hammond J, Carpenter K (2003) Screening nrwborns for inborn errors of metabolism by tandem mass-spectrometry. N Engl J Med 348:2304–2312
Wraith JE (2002) Lysosomal disorders. Semin Neonatol 7:75–83
Acknowledgements
We thank PerkinElmer, who provided the reagent kit and technical support for this study. We also thank Ray Hill, an independent medical writer on behalf of Springer Healthcare Communications, who provided medical writing support funded by Cometa A.S.M.M.E. – Associazione Studio Malattie Metaboliche Ereditarie – ONLUS. Sheridan Henness, PhD, of Springer Healthcare Communications assisted with post-submission revisions of the manuscript, which were funded by Cometa A.S.M.M.E. – Associazione Studio Malattie Metaboliche Ereditarie – ONLUS.
Funding
Medical writing support was funded by Cometa A.S.M.M.E. – Associazione Studio Malattie Metaboliche Ereditarie – ONLUS, Padova Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Alberto B Burlina has received speaker honoraria and travel support from Sanofi Genzyme, Biomarin and Actelion. He is a member of the European Advisory Board of Nutricia Danone and Biomarin.
Alessandro P Burlina has received speaker honoraria and travel support from Sanofi Genzyme and Amicus Therapeutics. He is a member of the European Advisory Board of the Fabry Registry, which is sponsored by Sanofi Genzyme.
Giulia Polo has received speaker honoraria and travel support from PerkinElmer and Actelion.
Bruno Bembi has received speaker honoraria and travel support from Sanofi Genzyme, and Actelion.
Giovanni Duro has received speaker honoraria, travel support and research grants from Sanofi Genzyme.
Robert J Desnick is a consultant for Sanofi Genzyme, Amicus Therapeutics, Alexion Pharmaceuticals, and Sangamo Therapeutics and receives research support from Sanofi Genzyme and Sangamo Therapeutics, and has founder stock in Amicus Therapeutics.
Leonardo Salviati, Roberta Zordan, Chiara Cazzorla, Laura Rubert, Carmela Zizzo, and Andrea Dardis, declare that they have no conflict of interest.
Additional information
Communicated by: Piero Rinaldo
Rights and permissions
About this article
Cite this article
Burlina, A.B., Polo, G., Salviati, L. et al. Newborn screening for lysosomal storage disorders by tandem mass spectrometry in North East Italy. J Inherit Metab Dis 41, 209–219 (2018). https://doi.org/10.1007/s10545-017-0098-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-017-0098-3