Abstract
Mucopolysaccharidosis type II (MPS II; Hunter syndrome; OMIM 309900) is a life-limiting, multisystemic disease with varying presentation and severity. Enzyme replacement therapy with intravenous idursulfase (EC 3.1.6.13) has been available since 2006. Data from the Hunter Outcome Survey (July 2016) were used to compare survival in idursulfase-treated (n = 800) and untreated (n = 95) male patients followed prospectively in this multinational, observational registry. Median age at symptom onset was similar for the treated and untreated groups (1.6 and 1.5 years, respectively), as was median age at diagnosis (3.3 and 3.2 years) and the proportion of patients with cognitive impairment (58.0%; 57.9%). The proportion of idursulfase-treated patients differed according to geographical region. Overall, 124/800 (15.5%) treated and 28/95 (29.5%) untreated patients had died. Respiratory failure was the most common cause of death (treated, 43/124 [34.7%]; untreated, 10/28 [35.7%]). Median survival (95% confidence interval [CI]) was 33.0 (30.4, 38.4) years in treated patients and 21.2 (16.1, 31.5) years in untreated patients; median follow-up time from birth to death or last visit was 13.0 and 15.1 years, respectively. A Cox model adjusted for treatment status, cognitive impairment, region and age at diagnosis indicated a 54% lower risk of death in treated compared with untreated patients: hazard ratio (HR), 0.46 (95% CI: 0.29, 0.72). Patients with cognitive impairment had nearly a fivefold higher risk of death than those without (HR, 4.84 [3.13, 7.47]). This analysis in a large population of patients with MPS II indicates for the first time that idursulfase treatment is associated with increased survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucopolysaccharidosis type II (MPS II; Hunter syndrome; OMIM 309900) is a rare, life-limiting, multisystemic, X-linked lysosomal storage disease. The disorder is caused by mutations in the IDS gene, which encodes the lysosomal enzyme iduronate-2-sulfatase (I2S; EC 3.1.6.13) (Neufeld and Muenzer 2001). Deficiency in I2S activity leads to gradual accumulation of glycosaminoglycans in organs and tissues throughout the body. The resulting signs and symptoms can include both somatic and neurological involvement and are progressive in nature (Neufeld and Muenzer 2001; Wraith et al 2008a; b). Those affected by MPS II are almost always males (Neufeld and Muenzer 2001), with an estimated incidence of 0.6–1.3 per 100,000 live male births (Meikle et al 1999; Poorthuis et al 1999; Baehner et al 2005; Tylki-Szymanska 2014).
Clinical presentation in patients with MPS II is highly variable and the disorder has a broad spectrum of severity (Neufeld and Muenzer 2001). For clinical purposes, patients are generally considered to be in one of two categories according to the presence or absence of cognitive impairment. In patients with cognitive impairment, somatic involvement is usually severe and of early onset and may include coarse facial features, bone and joint abnormalities, short stature, respiratory and cardiac disease and hearing abnormalities. Somatic involvement in patients without cognitive impairment can range from being severe with early onset to much less severe with later onset (Young et al 1982; Young and Harper 1983; Neufeld and Muenzer 2001; Schwartz et al 2007; Wraith et al 2008b); this group of patients usually exhibits minimal or no neurological involvement.
Management of patients with MPS II requires a multidisciplinary approach involving a range of specialties such as cardiology, neurology, psychology, pulmonology and orthopaedics (Wraith et al 2008a; Muenzer et al 2009; Scarpa et al 2011). Disease-specific treatment in the form of enzyme replacement therapy (ERT) with recombinant I2S (idursulfase [Elaprase®]; Shire, Lexington, MA, USA) became available in the USA in 2006 and Europe in 2007, and alleviates many of the somatic signs and symptoms of the disorder (Muenzer et al 2011); before this, symptomatic management was the only possible treatment approach.
The life expectancy of untreated patients with MPS II is considerably shorter than that in the general population. Individuals with severe cognitive impairment typically die in their second decade, usually as a result of respiratory obstruction and/or cardiac failure (Neufeld and Muenzer 2001; Wraith et al 2008b; Jones et al 2009). Those who experience only somatic involvement may survive into their fifth or sixth decade (Neufeld and Muenzer 2001; Wraith et al 2008b; Jones et al 2009). Survival has improved since 1985, hypothesized to be a result of improvements in supportive care and an increased awareness and understanding of the disease; the latter factors may have led to increased recognition of less severely affected patients and thus an increase in overall life expectancy (Jones et al 2009; Muenzer et al 2009; Scarpa et al 2011). The impact of ERT with idursulfase on survival and causes of death in patients with MPS II has not yet been evaluated.
The Hunter Outcome Survey (HOS) is a large multicentre, observational registry that was launched in 2005 to collect real-world data on the natural history of MPS II and the long-term safety and efficacy of ERT with idursulfase (Wraith et al 2008a, Muenzer et al 2017). The aim of this analysis was to compare survival in idursulfase-treated and untreated patients with MPS II. Causes of death were also evaluated.
Materials and methods
Registry design and data collection
HOS is designed to collect data on individuals with MPS II during routine patient visits and assessments (Wraith et al 2008b). All patients with a biochemically or genetically confirmed diagnosis of MPS II are eligible for enrolment; this includes those receiving intravenous idursulfase (or a bone marrow transplant [BMT]) and those who are not being treated. Individuals receiving treatment with an ERT product other than intravenous idursulfase are not eligible for enrolment. ‘Prospective patients’ are defined as those who are alive at study enrolment; if local regulations permit, deceased patients may also be enrolled (‘retrospective patients’) as described previously (Wraith et al 2008a; Jones et al 2009). A broad range of disease- and treatment-related information is captured in the registry, both prospectively and retrospectively (e.g. from medical histories). The study is observational in nature and the design does not include any predetermined assessments; patient visits and assessments occur as deemed appropriate by the managing physician.
Patient population
As of July 2016, 1200 patients were enrolled in HOS at 134 centres in 33 countries. Of these, 1034 were being followed prospectively. Two main groups of patients were included in this analysis: all male patients followed prospectively who had either received at least one dose of intravenous idursulfase (‘treated patients’) or who had never received intravenous idursulfase (‘untreated patients’). Patients who had received a BMT or who had died before their data were entered into the registry (retrospective patients) were excluded. Two individuals received treatment with intrathecally delivered idursulfase via participation in a clinical trial (SHP609-302, ClinicalTrials.gov identifier: NCT02412787) and so were no longer eligible to participate in HOS; for these patients, data after the date of surgical implantation of the intrathecal drug delivery device (IDDD) were not included in this analysis.
Data analysis
Demographic information, including age at onset of signs and symptoms, age at diagnosis, geographic region of residence, proportion of patients with cognitive impairment and number of patients who had died, was analysed for all patients in the treated and untreated groups for whom data were available. The presence of cognitive impairment was determined by the assessing healthcare professional on the basis of the answer to the yes/no question ‘Cognitive impairment?’ For the purposes of this analysis, a patient was considered to have cognitive impairment if the answer was recorded as ‘yes’ at any time; the answer to this question could have been based on clinical impression and/or standardized cognitive testing. Summary statistics of demographic data were calculated for the treated and untreated groups; no formal comparisons were made.
Causes of death recorded in the database were analysed in the treated and untreated groups. ‘Other’ is a category in the database in which causes of death not covered by the main database fields may be recorded using free text. The free text was reviewed by the authors (B.K.B., S.A.J.) from a medical perspective. In some cases, the cause of death was considered to fall under a main database category; in these instances, deaths originally listed as ‘Other’ were reclassified into the appropriate main category and the reclassification verified by the HOS Biostatistician (V.J.) and HOS Medical Monitor (Shire, Zug, Switzerland).
Survival time was defined as the time from date of birth to date of death. Date of birth was chosen as the reference point for both the treated and untreated groups because no common starting point was available for the start of intervention (treatment). Patients still alive at the time of the data extraction were censored at the last date on which they were known to be alive. Individuals who participated in the clinical study of intrathecal idursulfase were censored at the date of surgical implantation of the IDDD; these two patients had been on intravenous idursulfase for almost 4 years.
Kaplan–Meier analyses were used to estimate survival probability in the treated and untreated groups. Median patient follow-up time (from birth to last recorded visit) was also estimated for each group; patients may have been enrolled in the registry at any point during that period.
Multivariate Cox regression modelling was used to generate the hazard ratio (HR) for survival of treated versus untreated patients. The following variables were included in the primary Cox model: treatment status, presence of cognitive impairment and region of residence (as categorical covariates); and age at diagnosis (as a continuous covariate). Only patients with data available for all covariates were included in the model. The impact of each covariate on risk of death was also considered. Direct adjusted survival curves based on the primary Cox model were generated for the treated and untreated groups (Zhang et al 2007).
Additional sensitivity analyses utilizing Cox regression methodology were performed, including (a) investigation of the treatment effect on survival when patients with limited exposure to idursulfase were excluded (covariates used were the same as in the primary model; only patients who had received ERT for at least 6 months were included) and (b) the impact on survival of potential differences in supportive care. The latter was assessed by incorporating the occurrence of surgeries (yes/no) into the Cox model as covariates. Surgeries considered were adenoidectomy, cervical decompression, spinal fusion (HOS database categories, cervical/cervicolumbar fusion), gastrostomy/percutaneous endoscopic gastrostomy tube insertion, intracranial shunt placement/replacement, tonsillectomy, tracheotomy and valve replacement/repair. Choice of surgeries for inclusion was based on potential impact on survival and the degree to which performance of the procedure is likely to reflect the extent of overall patient care; surgeries commonly performed before diagnosis of MPS II were not included.
All analyses were performed using SAS® software version 9.3 (SAS Institute, Cary, NC, USA). Continuous data were summarized using medians and 10th, 90th percentiles (P10, P90).
Results
Patient characteristics
Table 1 displays the patient characteristics. In total, 895 male patients were included in this analysis: 800 treated and 95 untreated. The demographics of the treated and untreated groups were generally similar, with a median age (P10, P90) at symptom onset of 1.6 (0.3, 4.3) and 1.5 (0.2, 4.2) years, and a median age at diagnosis of 3.3 (1.0, 7.1) and 3.2 (0.9, 10.8) years, respectively. Median age at HOS entry was 7.9 (2.6, 21.1) years for the treated patients and 10.1 (2.8, 21.0) years for the untreated patients. Treated patients first received idursulfase at a median age of 6.9 (2.1, 19.8) years; median ERT duration was 57.8 (10.6, 106.2) months. The proportion of idursulfase-treated patients differed according to geographical region (Table 1); overall, most patients were from Europe and North America irrespective of treatment status. Similar proportions of treated and untreated patients had cognitive impairment at any time: 58.0% and 57.9%, respectively (Table 1).
Of the individuals included in the analysis, 124 treated patients (15.5%) and 28 untreated patients (29.5%) had died (Table 1). The most common cause of death was respiratory failure (treated patients, 43/124 [34.7%]; untreated patients, 10/28 [35.7%]). Other common causes of death in both groups were cardiac arrest and pneumonia (Fig. 1). Eight treated patients (6.5%) and one untreated patient (3.6%) had causes of death that were classified as ‘other’; these included malnutrition/dehydration, haemorrhage and neurological complications. The cause was classified in HOS as ‘unknown’ for 31 treated patients (25.0%) and 10 untreated patients (35.7%).
Causes of death in idursulfase-treated (n = 124/800) and untreated patients (n = 28/95). ‘Other’ is a category in the HOS database in which causes of death not covered by the main database fields may be recorded using free text. The free text was reviewed by the authors (B.K.B., S.A.J.) from a medical perspective. In some cases, the cause of death recorded in the ‘Other’ category was considered to fall under a main database category; in these instances, deaths originally listed as ‘Other’ were reclassified into the appropriate main category and the reclassification verified by the HOS Biostatistician (V.J.) and HOS Medical Monitor. ‘Unknown’ is a category for cause of death that may be selected from a pre-specified field in the database. Patient numbers in each category are shown on the bars. HOS, Hunter Outcome Survey
Patient survival
Kaplan–Meier analyses
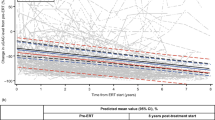
Median survival based on Kaplan–Meier estimates (95% confidence interval [CI]) in treated and untreated patients was 33.0 (30.4, 38.4) years and 21.2 (16.1, 31.5) years, respectively (Fig. 2A). Median (95% CI) follow-up time from birth to death or last-recorded visit was 13.0 (12.3, 13.8) years in treated patients and 15.1 (11.1, 18.8) years in untreated patients.
Survival curves for idursulfase-treated and untreated patients. a) Kaplan–Meier survival analysis for treated and untreated patients. b) Direct adjusted survival curves of predicted values for treated and untreated patients based on the primary Cox regression model. Multivariate Cox regression modelling for risk of death, adjusting for treatment status, cognitive impairment, geographical region of residence, and age at diagnosis. Survival was defined as the period from birth to date of death; for patients who were alive at the time of this analysis, censoring was performed at the last-recorded study visit
Multivariate Cox regression modelling
The adjusted HR for risk of death for treated versus untreated patients obtained from the primary Cox regression model was 0.46 (95% CI: 0.29, 0.72) (Table 2), corresponding to a 54% lower risk of death for treated patients compared with untreated patients; 113/695 treated patients and 28/81 untreated patients included in the model had died. Conversely, for untreated patients, the risk of death was approximately double that of treated patients. The increased probability for survival in the treated compared with the untreated group is illustrated in the adjusted survival curves (Fig. 2b); adjustment for the various covariates accounts for the smaller difference in median survival time between the two groups than is seen in the unadjusted Kaplan–Meier curves (Fig. 2a). Overall, the risk of death for patients from Latin America was approximately threefold greater than for those from North America (HR, 3.13 [95% CI: 1.83, 5.35]; Table 2); there was no significant difference for patients from other regions compared with North America. The risk of death was nearly fivefold higher in patients with cognitive impairment than in those without (adjusted for all covariates; HR, 4.84 [3.13, 7.47]).
Sensitivity analyses
Cox regression modelling was performed using the sub-cohort of patients who had received idursulfase for at least 6 months (a total of 747 treated and 94 untreated patients); 110/665 treated patients and 28/80 untreated patients included in the model had died. The results of this analysis supported the finding from the primary model of an impact of idursulfase on survival and indicated a 55% lower risk of death for treated than untreated patients (HR, 0.45 [0.29, 0.70]; adjusted for all covariates, data not shown).
Analysis using Cox regression modelling of the impact on survival of occurrence of surgeries throughout the follow-up period of this study indicated a slight but non-significant increase in risk of death for patients who had undergone surgeries compared with those who had not (HR, 1.21 [0.82, 1.78]; adjusted for all covariates from the primary model, data not shown; 695 treated and 81 untreated patients).
Discussion
In this first analysis of survival and causes of death in idursulfase-treated and untreated patients with MPS II, we demonstrate improved survival with intravenous idursulfase therapy. Multivariate Cox regression modelling indicated a 54% lower risk of death in treated than in untreated patients, and patients with cognitive impairment had nearly a fivefold higher risk of death than those without. The most common causes of death in patients in this analysis were respiratory failure, cardiac arrest and pneumonia.
There are a multitude of factors that may impact the relative clinical outcomes in treated and untreated patients with a complex, variable, multi-systemic disease such as MPS II. Among these are potential differences in overall clinical management and care between patients receiving ERT and untreated patients. Clinical characteristics or levels of disease severity may also differ between the two groups. However, the demographic characteristics of the treated and untreated patients in our analysis had a high degree of similarity, including the proportion of patients with cognitive impairment. The primary Cox model adjusted for potential confounding factors and for any variables that were imbalanced between the two groups (e.g. region). We explored the potential impact of differing levels of medical intervention and supportive care by including the number of surgeries as a covariate in a secondary model, but found no significant impact on survival. A further factor may be access to treatment. The primary Cox model indicated a greater risk of death in Latin America than in other regions; it is possible that this is a result of local challenges in accessing intravenous idursulfase therapy or specialist healthcare, although further investigation would be needed to confirm this. It should be noted that there is always the possibility of an impact on the study results from unmeasured variables.
There are several age-related covariates that could have been included in the primary Cox model. Of these, we chose age at diagnosis as this is well-defined and is typically well-recorded according to the date of the confirmatory biochemical or genetic test. In contrast, age at symptom onset may be based on recollection of previous signs and symptoms after formal diagnosis of MPS II; the date may not be clear cut and the judgement of the relationship of the symptom with MPS II may be subjective. Use of just one of these age-related covariates also resulted in inclusion of more patients in the primary Cox model, as well as eliminating any potential for co-linearity.
In this analysis, we also demonstrated that patients with cognitive impairment have a much higher risk of death than those without. This is consistent with a previous analysis of a smaller, retrospective, population from the HOS registry that showed that cognitive impairment substantially reduced survival (Jones et al 2009). It is important to note that the binary and subjective nature of the ‘Cognitive impairment? yes/no’ question in HOS results in some limitations, for example in capturing the extent of progressive cognitive decline. Nonetheless, categorization of patients on this basis provides useful insight into the occurrence of cognitive impairment and its impact on survival. Intravenously delivered idursulfase does not cross the blood–brain barrier in therapeutic concentrations and would not be expected to address the cognitive aspects of disease directly (Muenzer et al 2006; Muenzer et al 2012). The most common causes of death in our analysis (respiratory failure, cardiac arrest and pneumonia) had a somatic component; in addition, patients with neurological involvement may also have severe somatic disease. The multisystemic and progressive nature of MPS II means that a single cause of death is unlikely to reflect the full clinical picture in each patient. Nonetheless, the contribution of somatic aspects of disease to mortality is consistent with the positive impact of intravenous idursulfase on survival; approaches that aim to deliver therapeutic enzyme to the central nervous system are in development (Noh and Lee 2014; Muenzer et al 2016).
Registries such as HOS are especially valuable for rare diseases: the ability to follow a large number and broad spectrum of patients for a long period of time enables us to build on the knowledge gained in the formal clinical studies that are often restricted in scope (Muenzer et al 2017). However, registry data have some inherent limitations. For example, all data are collected during routine clinical evaluations and so there will be unavoidable differences between sites in the frequency of follow-up visits, the investigations performed at each visit and in standardization of the data collected (Muenzer et al 2017). The nature of the study population meant that various definitions of survival time were considered for use in this analysis; the period from birth to death or last-recorded clinic visit was chosen because there was no common variable for the start of intervention (intravenous idursulfase therapy). Although this approach does not adjust for time not receiving ERT, it is more robust than using other starting points, such as HOS entry. It should also be noted that it is possible for patients to remain enrolled in the registry but be lost to follow-up and with their survival status at the time of the data extract therefore not recorded. Loss to follow-up in the analysis population was minimized by requesting updated information from the sites with patients for whom more than 3 years had elapsed between the most recent recorded visit and the date of data extraction, to ensure that updated information was entered in as many instances as possible. We did observe a greater loss to follow-up in the untreated than in the treated group; this imbalance between the groups perhaps reflects that patients receiving intravenous idursulfase visit the clinic more frequently and are more closely monitored than those who are not receiving infusions. As with any registry, it was not possible to control the size of each patient group. In addition, randomization of patients to the treated and untreated groups, which would minimize channelling bias, is not possible with registry data. However, the patients included in this analysis are believed to be representative of the broader MPS II population and both groups are considered to be comparable.
Our analysis in patients with MPS II also adds considerably to our overarching understanding of ERT and survival in the broader context of all MPSs. Only limited data are available for MPS I and MPS VI (Dornelles et al 2014; Giugliani et al 2014); whilst these small studies indicate a possible survival benefit with ERT, our analysis is the first to show an impact in a large population of treated and untreated patients. Overall, despite the complexities of the data, our analysis demonstrates a survival benefit with intravenous idursulfase treatment in patients with MPS II.
Conclusions
We demonstrate for the first time increased survival with intravenous idursulfase treatment in a large population of patients with MPS II: median survival was 33.0 (30.4, 38.4) years in treated patients and 21.2 (16.1, 31.5) years in untreated patients, and an adjusted Cox model demonstrated a 54% lower risk of death (95% CI: 0.29, 0.72) for treated compared with untreated patients. These results provide a valuable addition to our understanding of the long-term benefits of intravenous idursulfase therapy in patients with this rare, debilitating disease.
References
Baehner F, Schmiedeskamp C, Krummenauer F et al (2005) Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis 28:1011–1017
Dornelles AD, de Camargo Pinto LL, de Paula AC et al (2014) Enzyme replacement therapy for Mucopolysaccharidosis type I among patients followed within the MPS Brazil network. Genet Mol Biol 37:23–29
Giugliani R, Lampe C, Guffon N et al (2014) Natural history and galsulfase treatment in mucopolysaccharidosis VI (MPS VI, Maroteaux-Lamy syndrome)--10-year follow-up of patients who previously participated in an MPS VI survey study. Am J Med Genet A 164A:1953–1964
Jones SA, Almassy Z, Beck M et al (2009) Mortality and cause of death in mucopolysaccharidosis type II-a historical review based on data from the Hunter Outcome Survey (HOS). J Inherit Metab Dis 32:534–543
Meikle PJ, Hopwood JJ, Clague AE, Carey WF (1999) Prevalence of lysosomal storage disorders. JAMA 281:249–254
Muenzer J, Beck M, Eng CM et al (2009) Multidisciplinary management of Hunter syndrome. Pediatrics 124:e1228–e1239
Muenzer J, Beck M, Eng CM et al (2011) Long-term, open-labeled extension study of idursulfase in the treatment of Hunter syndrome. Genet Med 13:95–101
Muenzer J, Jones SA, Tylki-Szymańska A et al (2017) Ten years of the Hunter Outcome Survey (HOS): insights, achievements, and lessons learned from a global patient registry. Orphanet J Rare Dis 12:82
Muenzer J, Bodamer O, Burton B et al (2012) The role of enzyme replacement therapy in severe Hunter syndrome-an expert panel consensus. Eur J Pediatr 171:181–188
Muenzer J, Hendriksz CJ, Fan Z et al (2016) A phase I/II study of intrathecal idursulfase-IT in children with severe mucopolysaccharidosis II. Genet Med 18:73–81
Muenzer J, Wraith JE, Beck M et al (2006) A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome). Genet Med 8:465–473
Neufeld EF, Muenzer J (2001) The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 3421–3452
Noh H, Lee JI (2014) Current and potential therapeutic strategies for mucopolysaccharidoses. J Clin Pharm Ther 39:215–224
Poorthuis BJ, Wevers RA, Kleijer WJ et al (1999) The frequency of lysosomal storage diseases in The Netherlands. Hum Genet 105:151–156
Scarpa M, Almassy Z, Beck M et al (2011) Mucopolysaccharidosis type II: European recommendations for the diagnosis and multidisciplinary management of a rare disease. Orphanet J Rare Dis 6:72
Schwartz IV, Ribeiro MG, Mota JG et al (2007) A clinical study of 77 patients with mucopolysaccharidosis type II. Acta Paediatr Suppl 96:63–70
Tylki-Szymanska A (2014) Mucopolysaccharidosis type II, Hunter’s syndrome. Pediatr Endocrinol Rev 12(Suppl 1):107–113
Wraith JE, Beck M, Giugliani R et al (2008a) Initial report from the Hunter Outcome Survey. Genet Med 10:508–516
Wraith JE, Scarpa M, Beck M et al (2008b) Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur J Pediatr 167:267–277
Young ID, Harper PS (1983) The natural history of the severe form of Hunter’s syndrome: a study based on 52 cases. Dev Med Child Neurol 25:481–489
Young ID, Harper PS, Newcombe RG, Archer IM (1982) A clinical and genetic study of Hunter’s syndrome. 2. Differences between the mild and severe forms. J Med Genet 19:408–411
Zhang X, Loberiza FR, Klein JP, Zhang MJ (2007) A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Prog Biomed 88:95–101
Acknowledgements
The authors would like to thank all those involved in HOS for their valuable contributions, in particular the patients enrolled in HOS and their families, as well as the HOS Investigators and study coordinators.
Disclosures/source of funding statement
This study was sponsored and funded by Shire, Lexington, MA, USA. Data collection and analysis are supported by Shire. Data analyses were performed by Shire under the direction of the authors. No honoraria, grants or other forms of payment were paid to the authors for the writing of the manuscript. Medical writing support was provided by Dr. Catriona Scott and Dr. Helen Bremner of Oxford PharmaGenesis, Oxford, UK and was funded by Shire, Zug, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
Barbara K. Burton has received consulting fees from BioMarin, Sanofi-Genzyme, Hyperion Therapeutics, Nora Therapeutics, REGENXBIO, ArmaGen and Shire, and has performed contracted research for Alexion Pharmaceuticals, Inc., BioMarin, Cytonet LLC, Sanofi-Genzyme, Shire, Synageva BioPharma, ArmaGen and Ultragenyx Pharmaceutical Inc.
Virginie Jego is an employee of Cytel Inc. and has received consulting fees from Shire.
Jaromir Mikl is an employee of Shire.
Simon A. Jones has received honoraria for speaking engagements and assistance with travel to conferences from Shire; he is also engaged in ongoing research projects with Shire, Sanofi-Genzyme, BioMarin, Ultragenyx Pharmaceutical Inc. and Alexion Pharmaceuticals, Inc.
Ethics approval statement
Independent Review Board/Ethics Committee approval was obtained for all participating centres. HOS is conducted in accordance with the Guidelines for Good Pharmacoepidemiology Practices, Good Research for Comparative Effectiveness Principles and the relevant principles of the International Conference on Harmonisation Good Clinical Practice guidelines (ICH E6).
Informed consent
Each patient, their parents or a legal representative provided signed and dated written informed consent for participation in the Hunter Outcome Survey (HOS). All patient information is managed in accordance with national data protection standards.
Additional information
Responsible Editor: Marc Patterson
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Burton, B.K., Jego, V., Mikl, J. et al. Survival in idursulfase-treated and untreated patients with mucopolysaccharidosis type II: data from the Hunter Outcome Survey (HOS). J Inherit Metab Dis 40, 867–874 (2017). https://doi.org/10.1007/s10545-017-0075-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-017-0075-x