Abstract
Background
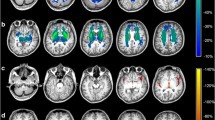
Glutaric aciduria type I (GA-I) is a cerebral organic aciduria caused by inherited deficiency of glutaryl-CoA dehydrogenase and is characterized biochemically by an accumulation of putatively neurotoxic dicarboxylic metabolites. The majority of untreated patients develops a complex movement disorder with predominant dystonia during age 3–36 months. Magnetic resonance imaging (MRI) studies have demonstrated striatal and extrastriatal abnormalities.
Aims/methods
The major aim of this study was to elucidate the complex neuroradiological pattern of patients with GA-I and to associate the MRI findings with the severity of predominant neurological symptoms. In 180 patients, detailed information about the neurological presentation and brain region-specific MRI abnormalities were obtained via a standardized questionnaire.
Results
Patients with a movement disorder had more often MRI abnormalities in putamen, caudate, cortex, ventricles and external CSF spaces than patients without or with minor neurological symptoms. Putaminal MRI changes and strongly dilated ventricles were identified as the most reliable predictors of a movement disorder. In contrast, abnormalities in globus pallidus were not clearly associated with a movement disorder. Caudate and putamen as well as cortex, ventricles and external CSF spaces clearly collocalized on a two-dimensional map demonstrating statistical similarity and suggesting the same underlying pathomechanism.
Conclusions
This study demonstrates that complex statistical methods are useful to decipher the age-dependent and region-specific MRI patterns of rare neurometabolic diseases and that these methods are helpful to elucidate the clinical relevance of specific MRI findings.



Similar content being viewed by others
Abbreviations
- FLAIR:
-
Fluid attenuated inversion recovery
- GA-I:
-
Glutaric aciduria type I (OMIM #231670)
- MD:
-
Movement disorder
- MDS:
-
Multi-dimensional scaling
- MRI:
-
Magnetic resonance imaging
References
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57:289–300
Bijarnia S, Wiley V, Carpenter K, Christodoulou J, Ellaway CJ, Wilcken B (2008) Glutaric aciduria type I: outcome following detection by newborn screening. J Inherit Metab Dis 31:503–507
Boneh A, Beauchamp M, Humphrey M, Watkins J, Peters H, Yaplito-Lee J (2008) Newborn screening for glutaric aciduria type I in Victoria: treatment and outcome. Mol Genet Metab 94:287–291
Busquets C, Merinero B, Christensen E et al (2000) Glutaryl-CoA dehydrogenase deficiency in Spain: evidence of two groups of patients, genetically, and biochemically distinct. Pediatr Res 48:315–322
Chow CW, Haan EA, Goodman SI et al (1988) Neuropathology in glutaric acidemia type 1. Acta Neuropathol 76:590–594
Couce ML, López-Suárez O, Bóveda MD et al (2013) Glutaric aciduria type I: outcome of patients with early- versus late-diagnosis. Eur J Paediatr Neurol 74:383–389
Funk CB, Prasad AN, Frosk P et al (2005) Neuropathological, biochemical, and molecular findings in a glutaric acidemia type 1 cohort. Brain 128:711–722
Gerstner B, Gratopp A, Marcinkowski M, Sifringer M, Obladen M, Buhrer C (2005) Glutaric acid and its metabolites cause apoptosis in immature oligodendrocytes: a novel mechanism of white matter degeneration in glutaryl-CoA dehydrogenase deficiency. Pediatr Res 57:771–776
Gitiaux C, Roze E, Kinugawa K et al (2008) Spectrum of movement disorders associated with glutaric aciduria type 1: a study of 16 patients. Mov Disord 23:2392–2397
Goodman SI, Markey SP, Moe PG, Miles BS, Teng CC (1975) Glutaric aciduria: a “new” disorder of amino acid metabolism. Biochem Med 12:12–21
Harting I, Neumaier-Probst E, Seitz A et al (2009) Dynamic changes of striatal and extrastriatal abnormalities in glutaric aciduria type I. Brain 132:1764–1782
Heringer J, Boy SPN, Ensenauer R et al (2010) Use of guidelines improves the neurological outcome in glutaric aciduria type I. Ann Neurol 68:743–752
Hothorn T, Hornik K, Zeileis A (2006) Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat 15:651–674
Janos AL, Grange DK, Steiner RD, White DA (2012) Processing speed and executive abilities in children with phenylketonuria. Neuropsychology 26:735–743
Kölker S, Sauer SW, Surtees RA, Leonard JV (2006a) The aetiology of neurological complications of organic acidaemias—a role for the blood–brain barrier. J Inherit Metab Dis 29:701–704
Kölker S, Garbade SF, Greenberg CR et al (2006b) Natural history, outcome and therapeutic efficacy in children and adults with glutaryl-CoA dehydrogenase deficiency. Pediatr Res 59:840–847
Kölker S, Garbade SF, Boy N et al (2007) Decline of acute encephalopathic crises in children with glutaryl-CoA dehydrogenase deficiency identified by newborn screening in Germany. Pediatr Res 62:357–363
Kölker S, Christensen E, Leonard JV et al (2011) Diagnosis and management of glutaric aciduria type I—revised recommendations. J Inherit Metab Dis 34:677–694
Kracun I, Rösner H, Cosovic C, Stavljenic A (1984) Topographical atlas of the gangliosides of the adult human brain. J Neurochem 43:979–989
Külkens S, Harting I, Sauer S et al (2005) Late-onset neurologic disease in glutaryl-CoA dehydrogenase deficiency. Neurology 64:2142–2144
Lamp J, Keyser B, Koeller DM, Ullrich K, Braulke T, Mühlhausen C (2011) Glutaric aciduria type 1 metabolites impair the succinate transport from astrocytes to neuronal cells. J Biol Chem 286:17777–17784
Lin SK, Hsu SG, Ho ES et al (2002) Novel mutations and prenatal sonographic findings of glutaric aciduria (type I) in two Taiwanese families. Prenat Diagn 22:725–729
Neumaier-Probst E, Harting I, Seitz A, Ding C, Kölker S (2004) Neuroradiological findings in glutaric aciduria type I (glutaryl-CoA dehydrogenase deficiency). J Inherit Metab Dis 27:869–876
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Pfeil J, Listl S, Hoffmann GF, Kölker S, Lindner M, Burgard P (2013) Newborn screening by tandem mass spectrometry for glutaric aciduria type 1: a cost-effectiveness analysis. Orphanet J Rare Dis 8:167
R Development Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, ISBN 3-900051-07-0, URL http://www.R-project.org
Sauer SW, Okun JG, Schwab MA et al (2005) Bioenergetics in glutaryl-coenzyme A dehydrogenase deficiency, a role for glutaryl-coenzyme A. J Biol Chem 280:21830–21836
Sauer SW, Okun JG, Fricker G et al (2006) Intracerebral accumulation of glutaric and 3-hydroxyglutaric acids in glutaryl-coenzyme A dehydrogenase deficiency, a biochemical risk factor for neurodegeneration. J Neurochem 97:899–910
Sauer SW, Opp S, Mahringer A et al (2010) Glutaric aciduria type I and methylmalonic aciduria: simulation of cerebral import and export of accumulating neurotoxic dicarboxylic acids in in vitro models of the blood–brain barrier and the choroid plexus. Biochim Biophys Acta 1802:552–560
Sauer SW, Opp S, Hoffmann GF, Koeller DM, Okun JG, Kölker S (2011) Therapeutic modulation of cerebral L-lysine metabolism in a mouse model for glutaric aciduria type I. Brain 134:157–170
Soffer D, Amir N, Elpeleg ON et al (1992) Striatal degeneration and spongy myelinopathy in glutaric acidemia. J Neurol Sci 107:199–204
Strauss KA, Lazovic J, Wintermark M, Morton DH (2007) Multimodal imaging of striatal degeneration in Amish patients with glutaryl-CoA dehydrogenase deficiency. Brain 130:1905–1920
Strauss KA, Donnelly P, Wintermark M (2010) Cerebral haemodynamics in patients with glutaryl-coenzyme A dehydrogenase deficiency. Brain 133:76–92
Twomey EL, Naughten ER, Donoghue VB, Ryan S (2003) Neuroimaging findings in glutaric aciduria type 1. Pediatr Radiol 33:823–830
Van der Knaap MS, Valk J (2005) Myelination and retarded myelination. In: Van der Knaap MS, Valk J (eds) Magnetic resonance of myelination and myelin disorders, 3rd edn. Springer-Verlag, Berlin, pp 37–65
Viau K, Ernst SL, Vanzo RJ, Botto LD, Pasquali M, Longo N (2012) Glutaric acidemia type 1: outcomes before and after expanded newborn screening. Mol Genet Metab 106:430–438
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Zinnanti WJ, Lazovic J, Housman C et al (2007) Mechanism of age-dependent susceptibility and novel treatment strategy in glutaric acidemia type I. J Clin Invest 117:3258–3270
Zschocke J, Quak E, Guldberg P, Hoffmann GF (2000) Mutation analysis in glutaric aciduria type I. J Med Genet 37:177–181
Acknowledgments
We are indebted to the patients and their families for their participation and trust. We thank the patient advocacy group “Glutarazidurie e.V.” (www.glutarazidurie.de) for excellent collaboration.
This study was supported by a grant from the “Kindness for Kids” Foundation, Munich, Germany to S. K. This is a private foundation focusing on stimulating research for children with rare diseases.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Nicole Wolf
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Fig. 1
Histogram of age of the GA-I study cohort. (JPEG 101 kb)
Suppl. Fig. 2
Multidimensional scaling of neuroradiological items using the minimum-spanning tree in a group of patients older than three years. The stress was 9.06%. Collocalization of pathologic MRI changes in ventricles, external CSF spaces and cortex, as well as in caudate and putamen indicate similarities in the underlying mechanisms. Globus pallidus, a white-matter rich grey matter structure, is located between caudate/putamen and white matter indicating that pallidal MRI changes may reflect putatively reversible white matter pathology or irreversible basal ganglia damage. Results are almost identical to an analysis of the whole study group (see Fig. 3). (JPEG 110 kb)
Suppl. Figs. 3–7
Age-dependent frequency and severity of MRI changes in putamen (Suppl. Fig. 3), caudate (Suppl. Fig. 4), white matter (Suppl. Fig. 5), pallidum (Suppl. Fig. 6), and thalamus (Suppl. Fig. 7) was depicted as spinograms. Striatal MRI abnormalities reflect an age-dependent window of vulnerability of this brain region during the youngest age group, whereas the frequency and severity of pallidal MRI changes were similar in all age groups. MRI abnormalities in the white showed some increase in older compared to younger age groups. MRI abnormalities in other brain regions such as thalamus were only rarely detected. (JPEG 94 kb)
Suppl. Table 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Garbade, S.F., Greenberg, C.R., Demirkol, M. et al. Unravelling the complex MRI pattern in glutaric aciduria type I using statistical models—a cohort study in 180 patients. J Inherit Metab Dis 37, 763–773 (2014). https://doi.org/10.1007/s10545-014-9676-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-014-9676-9