Abstract
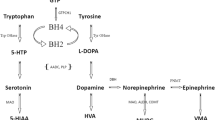
Smith-Lemli-Opitz syndrome (SLOS) is an autosomal recessive, multiple congenital anomaly syndrome with cognitive impairment and a distinct behavioral phenotype that includes autistic features. SLOS is caused by a defect in 3β-hydroxysterol Δ7-reductase which leads to decreased cholesterol levels and elevated cholesterol precursors, specifically 7- and 8-dehydrocholesterol. However, the pathological processes contributing to the neurological abnormalities in SLOS have not been defined. In view of prior data suggesting defects in SLOS in vesicular release and given the association of altered serotonin metabolism with autism, we were interested in measuring neurotransmitter metabolite levels in SLOS to assess their potential to be used as biomarkers in therapeutic trials. We measured cerebral spinal fluid levels of serotonin and dopamine metabolites, 5-hydroxyindoleacetic acid (5HIAA) and homovanillic acid (HVA) respectively, in 21 SLOS subjects. Results were correlated with the SLOS anatomical severity score, Aberrant Behavior Checklist scores and concurrent sterol biochemistry. Cerebral spinal fluid (CSF) levels of both 5HIAA and HVA were significantly reduced in SLOS subjects. In individual patients, the levels of both 5HIAA and HVA were reduced to a similar degree. CSF neurotransmitter metabolite levels did not correlate with either CSF sterols or behavioral measures. This is the first study demonstrating decreased levels of CSF neurotransmitter metabolites in SLOS. We propose that decreased levels of neurotransmitters in SLOS are caused by a sterol-related defect in synaptic vesicle formation and that CSF 5HIAA and HVA will be useful biomarkers in development of future therapeutic trials.

Similar content being viewed by others
References
Aman MG, Singh NN, Stewart AW, Field CJ (1985) Psychometric characteristics of the aberrant behavior checklist. Am J Ment Defic 89(5):492–502
Bartholomeusz HH, Courchesne E, Karns CM (2002) Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics 33(5):239–241
Caruso PA, Poussaint TY, Tzika AA et al (2004) MRI and 1H MRS findings in Smith-Lemli-Opitz syndrome. Neuroradiology 46(1):3–14
Fantini J, Barrantes FJ (2009) Sphingolipid/cholesterol regulation of neurotransmitter receptor conformation and function. Biochim Biophys Acta 1788(11):2345–2361
Fitzky BU, Witsch-Baumgartner M, Erdel M et al (1998) Mutations in the Delta7-sterol reductase gene in patients with the Smith-Lemli-Opitz syndrome. Proc Natl Acad Sci U S A 95(14):8181–8186
Fliesler SJ, Richards MJ, Miller C, Peachey NS (1999) Marked alteration of sterol metabolism and composition without compromising retinal development or function. Invest Ophthalmol Vis Sci 40(8):1792–1801
Gondre-Lewis MC, Petrache HI, Wassif CA et al (2006) Abnormal sterols in cholesterol-deficiency diseases cause secretory granule malformation and decreased membrane curvature. J Cell Sci 119(Pt 9):1876–1885
Hyland K (2008) Clinical utility of monoamine neurotransmitter metabolite analysis in cerebrospinal fluid. Clin Chem 54(4):633–641
Hyland K, Surtees RA, Heales SJ, Bowron A, Howells DW, Smith I (1993) Cerebrospinal fluid concentrations of pterins and metabolites of serotonin and dopamine in a pediatric reference population. Pediatr Res 34(1):10–14
Irons M, Elias ER, Salen G, Tint GS, Batta AK (1993) Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet 341(8857):1414
Kelley RI (1995) Diagnosis of Smith-Lemli-Opitz syndrome by gas chromatography/mass spectrometry of 7-dehydrocholesterol in plasma, amniotic fluid and cultured skin fibroblasts. Clin Chim Acta 236(1):45–58
Kelley RI, Hennekam RC (2000) The Smith-Lemli-Opitz syndrome. J Med Genet 37(5):321–335
Krakowiak PA, Nwokoro NA, Wassif CA et al (2000) Mutation analysis and description of sixteen RSH/Smith-Lemli-Opitz syndrome patients: polymerase chain reaction-based assays to simplify genotyping. Am J Med Genet 94(3):214–227
Lee RW, Conley SK, Gropman A, Porter FD, Baker EH (2013a) Brain magnetic resonance imaging findings in smith-lemli-opitz syndrome. Am J Med Genet A 161:2407–2419
Lee RW, Yoshida S, Jung ES, Mori S, Baker EH, Porter FD (2013b) Corpus callosum measurements correlate with developmental delay in smith-lemli-opitz syndrome. Pediatr Neurol 49(2):107–112
Mueller C, Patel S, Irons M et al (2003) Normal cognition and behavior in a Smith-Lemli-Opitz syndrome patient who presented with Hirschsprung disease. Am J Med Genet A 123A(1):100–106
Narayan M, Srinath S, Anderson GM, Meundi DB (1993) Cerebrospinal fluid levels of homovanillic acid and 5-hydroxyindoleacetic acid in autism. Biol Psychiatry 33(8–9):630–635
Paila YD, Murty MR, Vairamani M, Chattopadhyay A (2008) Signaling by the human serotonin(1A) receptor is impaired in cellular model of Smith-Lemli-Opitz Syndrome. Biochim Biophys Acta 1778(6):1508–1516
Paila YD, Tiwari S, Sengupta D, Chattopadhyay A (2011) Molecular modeling of the human serotonin(1A) receptor: role of membrane cholesterol in ligand binding of the receptor. Mol Biosyst 7(1):224–234
Porter FD (2000) RSH/Smith-Lemli-Opitz syndrome: a multiple congenital anomaly/mental retardation syndrome due to an inborn error of cholesterol biosynthesis. Mol Genet Metab 71(1–2):163–174
Porter FD (2008) Smith-Lemli-Opitz syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet 16(5):535–541
Rosa P, Fratangeli A (2010) Cholesterol and synaptic vesicle exocytosis. Commun Integr Biol 3(4):352–353
Ryan AK, Bartlett K, Clayton P et al (1998) Smith-Lemli-Opitz syndrome: a variable clinical and biochemical phenotype. J Med Genet 35(7):558–565
Scott MM, Deneris ES (2005) Making and breaking serotonin neurons and autism. Int J Dev Neurosci 23(2–3):277–285
Shrivastava S, Pucadyil TJ, Paila YD, Ganguly S, Chattopadhyay A (2010) Chronic cholesterol depletion using statin impairs the function and dynamics of human serotonin(1A) receptors. Biochemistry 49(26):5426–5435
Singh P, Paila YD, Chattopadhyay A (2007) Differential effects of cholesterol and 7-dehydrocholesterol on the ligand binding activity of the hippocampal serotonin(1A) receptor: implications in SLOS. Biochem Biophys Res Commun 358(2):495–499
Sjogren B, Csoregh L, Svenningsson P (2008) Cholesterol reduction attenuates 5-HT1A receptor-mediated signaling in human primary neuronal cultures. Naunyn Schmiedebergs Arch Pharmacol 378(4):441–446
Smith DW, Lemli L, Opitz JM (1964) A newly recognized syndrome of multiple congenital anomalies. J Pediatr 64:210–217
Surtees R, Heales S, Bowron A (1994) Association of cerebrospinal fluid deficiency of 5-methyltetrahydrofolate, but not S-adenosylmethionine, with reduced concentrations of the acid metabolites of 5-hydroxytryptamine and dopamine. Clin Sci (Lond) 86(6):697–702
Tierney E, Nwokoro NA, Porter FD, Freund LS, Ghuman JK, Kelley RI (2001) Behavior phenotype in the RSH/Smith-Lemli-Opitz syndrome. Am J Med Genet 98(2):191–200
Tierney E, Conley SK, Goodwin H, Porter FD (2010) Analysis of short-term behavioral effects of dietary cholesterol supplementation in Smith-Lemli-Opitz syndrome. Am J Med Genet A 152A(1):91–95
Waage-Baudet H, Lauder JM, Dehart DB et al (2003) Abnormal serotonergic development in a mouse model for the Smith-Lemli-Opitz syndrome: implications for autism. Int J Dev Neurosci 21(8):451–459
Wassif CA, Maslen C, Kachilele-Linjewile S et al (1998) Mutations in the human sterol delta7-reductase gene at 11q12-13 cause Smith-Lemli-Opitz syndrome. Am J Hum Genet 63(1):55–62
Wassif CA, Zhu P, Kratz L et al (2001) Biochemical, phenotypic and neurophysiological characterization of a genetic mouse model of RSH/Smith–Lemli–Opitz syndrome. Hum Mol Genet 10(6):555–564
Waterham HR, Wanders RJ (2000) Biochemical and genetic aspects of 7-dehydrocholesterol reductase and Smith-Lemli-Opitz syndrome. Biochim Biophys Acta 1529(1–3):340–356
Acknowledgements and funding support
This work was supported by the intramural program of the National Institute of Child Health and Human Development and a Bench-to-Bedside award from the Office of Rare Diseases and the NIH Clinical Center. Autism Speaks, the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1 TR 000424-06 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS or NIH. We would like to thank Richard Kelly for critical review of this manuscript. We would like to express our appreciation to the patients and families that participated in this study.
Competing interest
Susan Sparks, Chris Wassif, Halima Goodwin, Sandra Conley, Diane Lanham, Andrea Gropman, Elaine Tierney and Forbes Porter report no conflicts of interest.
Lisa Kratz is employed in the Biochemical Genetics Laboratory, Kennedy Krieger Institute, Baltimore, MD. This laboratory performs clinical testing for sterols.
Keith Hyland is employed by Medical Neurogenetics, Atlanta, GA. Medical Neurogenetics performs commercial clinical testing of CSF neurotransmitter metabolites.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: K. Michael Gibson
Rights and permissions
About this article
Cite this article
Sparks, S.E., Wassif, C.A., Goodwin, H. et al. Decreased cerebral spinal fluid neurotransmitter levels in Smith-Lemli-Opitz syndrome. J Inherit Metab Dis 37, 415–420 (2014). https://doi.org/10.1007/s10545-013-9672-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-013-9672-5