Abstract
Objective
The Suspicion Index (SI) screening tool was developed to identify patients suspected of having Niemann-Pick disease type C (NP-C). The SI provides a risk prediction score (RPS) based on NP-C manifestations within and across domains (visceral, neurological, and psychiatric). The aim of these subanalyses was to further examine the discriminatory power of the SI by age and manifestation–associations by NP-C suspicion-level and leading manifestations.
Methods
The original retrospectively collected data were split into three patient age groups, where NP-C-positive cases were >16 years (n = 30), 4–16 years (n = 18), and <4 years (n = 23), and patients’ RPS were analyzed by logistic regression. Co-occurrence of manifestations within groups of suspicion level (low, medium, high) and leading manifestations (presence/absence of ataxia, cognitive decline, psychosis, and splenomegaly) were analyzed descriptively.
Results
NP-C-positive cases versus controls showed strong discriminatory power of RPS. Area under the receiver operating characteristic curve was 0.964 (>16 years) and 0.981 (4–16 years) but weaker 0.562 for infants (<4 years). Patients with RPS <70 were characterized by a lack of psychiatric manifestations and low levels of neurological involvement, suggestive of a preneurological phase of the disease. In patients >4 years, prominent leading manifestation–associations were ataxia with dystonia, dysarthria/dysphagia, and cognitive decline. Psychosis was associated with dysarthria/dysphagia but also with cognitive decline and treatment-resistant psychiatric symptoms.
Conclusions
The SI tool maintains strong discriminatory power in patients >4 years but is not as useful for infants <4 years. The SI is also informative regarding the association and co-occurrence of manifestations in patients with NP-C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Niemann-Pick disease type C (NP-C) is a rare, autosomal recessive disease caused by mutations in the NPC1 (95 % of cases) or NPC2 genes. The disease is characterized by heterogeneous and oligosymptomatic presentation of visceral, neurological, and psychiatric manifestations, making for difficult and often delayed diagnosis (Patterson et al. 2012; Vanier 2010; Wraith et al. 2009; Wraith and Imrie 2009). Current estimated incidence is 0.85 per 100,000 live births (Orphanet Report Series 2011).
Diagnosis of NP-C is made via physical assessment of the patient, biochemical tests involving filipin staining of skin fibroblasts, and genetic sequencing of the NPC1 and NPC2 mutations (Wraith et al. 2009; Vanier 2010; Patterson et al. 2012). Filipin staining and genetic analysis are recommended as the first-line diagnostic tests, to be carried out in parallel if possible in order to obtain complementary information (Patterson et al. 2012). However, the choice of first-line test depends on the local availability of techniques and expertise.
Miglustat, the first and currently only specific treatment for pediatric and adult patients with NP-C, was approved for use in the European Union in 2009 (Zavesca®, Actelion Pharmaceuticals Ltd, Allschwil, Switzerland) (European Medicines Agency 2012; Patterson et al. 2012) and in Japan in April 2012 (Brazaves®). Early diagnosis of NP-C is essential so that uninterrupted miglustat therapy can start at the onset of neurological manifestations (Wraith et al. 2010; Wraith et al. 2009; Pineda et al. 2010; Vanier 2010; Wraith and Imrie 2009; Chien et al. 2012).
The NP-C Suspicion Index (SI) screening tool, developed by an international panel of NP-C clinical experts, aids physicians unfamiliar with NP-C in early identification of patients with suspicion of NP-C (www.NPC-SI.com; Wijburg et al. 2012). A risk prediction score (RPS) is calculated by the presence of key clinical manifestations of NP-C within and across three domains plus family history information. Due to the disease variability at age of onset and presentation, it is important to determine whether the SI tool performs in different patient groups. This manuscript reports subanalyses of the original study data to investigate the discriminatory power of the SI tool by age and to investigate associations by the presence and absence of four leading NP-C manifestations.
Methods
Subanalyses were performed on data arising from a published study reporting the development of the NP-C SI tool (Wijburg et al. 2012). The published study involved a retrospective chart review of 216 patients in seven specialist NP-C centers in Europe and Australia. Data were collected from two patient groups who were tested for NP-C: 71 NP-C-positive cases, confirmed by classical or variant NP-C filipin staining, with subsequent identification of at least one mutation in NPC1 or NPC2 genes; and 64 suspected NP-C cases but in whom filipin staining was negative. Data for NP-C-positive cases and suspected cases were collected up to the decision to refer for a filipin test. Data were also collected from 81 control patients who were without suspicion for NP-C but who presented with at least one symptom associated with NP-C and, where possible, from the same outpatient clinic as the NP-C-positive and suspected cases.
Collected data included demographics such as age and gender, presence of individual manifestations within the three domains (visceral, neurological, and psychiatric), and patient’s first- or second-degree family history. The scoring system of the SI tool assigned points to individual NP-C manifestations within each domain. In addition, points were assigned for manifestations presenting across two or three domains and for familial history. The sum of all points gave the total RPS for each patient. RPS ≥70 indicated high suspicion of NP-C and recommendation for immediate testing at an NP-C specialist center. RPS score between 40 and 69 indicated moderate suspicion for NP-C and recommendation for follow-up observation as well as further discussion with an NP-C referral center. RPS <40 indicated a low probability of NP-C.
Subanalyses involved categorization of all patients by age: infantile patients < 4 years (n = 23 for NP-C-positive patients, n = 23 for NP-C-suspected cases, and n = 7 for control patients), juvenile patients 4–16 years (n = 18 for NP-C-positive patients, n = 16 for NP-C-suspected patients, and n = 39 for control patients), and adolescent patients >16 years (n = 30 for NP-C-positive patients, n = 25 for NP-C-suspected patients, and n = 35 for control patients). Frequency distribution of manifestations within and across domains was tabulated for each patient age group. The performance of the total and individual RPS for each domain was investigated via logistic regression within each age group using the outcome NP-C-positive cases versus combined NP-C-suspected cases and controls as binary dependent variables and the RPS as an independent variable. Receiver operating characteristic (ROC) curve (sensitivity versus 1-specificity) analysis was performed, and the area under the curve (AUC) was estimated. Frequency distribution of manifestations by RPS (disease severity) categories < 70 points, 70–150 points, and >150 points was tabulated and also presented graphically to show the scatter of RPS by age, where age was transformed to a logarithmic scale to improve visual assessment in the infantile group.
Association (co-occurrence) of manifestations by the presence and absence of four leading NP-C manifestations—ataxia, cognitive decline, psychosis, and splenomegaly—were examined descriptively by frequency distribution of manifestations and graphical presentation in patients >4 years. These leading manifestations are moderate or strong indicators for NP-C in each domain, and due to their high frequency of occurrence, the presence of one or more of these manifestations is most often the reason for referral for NP-C testing. Other specific NP-C symptoms, such as vertical supranuclear gaze palsy (VSGP), cataplexy, and epilepsy, were excluded as leading symptoms because their presence is rarely the initial cause for referral for NP-C testing due to their lower frequency, generally later onset, and—in some cases such as VSGP—difficulty of diagnosis. These data were analyzed descriptively.
Results
Frequency and association of manifestations by age group
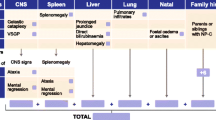
The most frequent (>50 %) manifestations displayed in infantile patients (<4 years of age) were prolonged neonatal jaundice, splenomegaly, and delayed developmental milestones. Hypotonia occurred mainly in infantile patients but not in older patients. Infantile patients demonstrated low levels of psychiatric manifestations and reduced levels of neurological involvement. The majority of manifestations in this patient group appeared across the visceral and neurological domains (Fig. 1).
Visceral manifestations were less frequent in adolescent patients (>16 years of age), as shown in Fig. 1; however, findings within the neurological domain, in particular, VSGP, dystonia, and dysarthria/dysphagia, became more frequent. The frequency of cognitive decline, a strong psychiatric indicator of NP-C, noticeably increased in juvenile and adolescent patients. The frequency of other manifestations within the psychiatric domain, including psychotic symptoms, treatment-resistant psychiatric symptoms, disruptive or aggressive behavior, and other psychiatric disorders also increased in patients >16 years of age. As in the infantile patient group, juvenile and adolescent patients displayed manifestations clustered across the visceral and neurological domains. Additionally, patients >4 years of age displayed an increased frequency of combined manifestations across the visceral/psychiatric, visceral/neurological, and neurological/psychiatric domains.
Frequency and association of manifestations by RPS
Patients with a total RPS <70 points demonstrated a noticeable lack of psychiatric findings and lower levels of neurological involvement, indicating a more visceral phenotype (Fig. 2).
Increased presence of VSGP, splenomegaly, ataxia, and all psychiatric manifestations were associated with high suspicion of NP-C (RPS >150 points). Increased frequency of manifestations both within the same category, but more so across categories, greatly increased the RPS. The mean RPS for infantile patients <4 years of age was much lower than RPS for juvenile and adolescent patients (mean RPS of 79.2 points compared with 190 and 179, respectively, Fig. 3). As shown in Fig. 4, patients with an RPS score of <70 tended to be infantile patients <4 years of age.
Association by presence/absence of leading manifestations in patients >4 years
The leading neurological manifestation of ataxia (n = 41, 85.4 % of patients), a moderate indicator of NP-C according to the SI tool, most commonly occurred with other manifestations within the same category, such as dystonia and dysarthria/dysphagia, but also with cognitive decline, which is a psychiatric domain manifestation (Fig. 5). The two leading psychiatric manifestations were cognitive decline (a strong indicator of NP-C) and psychosis (a moderate indicator of NP-C). NP-C-positive patients showing manifestations of cognitive decline (n = 42, 87.5 %) displayed increased levels of psychosis and slightly elevated frequency of treatment-resistant psychiatric symptoms, which are within the same domain category. However, cross-domain manifestations in the neurological category were also observed. These symptoms include ataxia, gelastic cataplexia, dystonia, and seizures (Fig. 5). Patients without cognitive decline showed increased findings of other types of psychiatric disorders. Psychosis (n = 15, 31.3 %) was associated with other illness features, including treatment-resistant psychiatric symptoms and across-category neurological findings, including dysarthria/dysphagia and dystonia (Fig. 6).
Manifestation–association in patients ≥4 years with Niemann-Pick disease type C (NP-C) by presence/absence of ataxia and cognitive decline. Frequency (%) of positive NP-C manifestations in each of three domains (visceral, neurological, and psychiatric) associated with and without ataxia and with and without cognitive decline (n = 48)
Manifestation–association in patients ≥4 years with Niemann-Pick disease type C (NP-C) by presence/absence of psychosis and splenomegaly. Frequency (%) of positive NP-C manifestations in each of the three domains (visceral, neurological, and psychiatric) associated with and without psychosis and with and without splenomegaly (n = 48)
The leading manifestation in the visceral domain was splenomegaly (n = 26, 54.2 %), a strong indicator of NP-C. There was little difference between manifestation–association within and across domains for patients with or without splenomegaly (Fig. 6). Patients without splenomegaly (n = 22) showed a slight increase in frequency of prolonged neonatal jaundice, ataxia, and dysarthria/dysphagia compared with patients with splenomegaly.
Discriminatory power by age
Discriminatory power of the NP-C SI for NP-C-positive cases versus combined NP-C-suspected cases and controls was stronger for juvenile (aged 4–16 years) and adolescent (>16 years of age) patients than for infantile (<4 years of age) patients. The estimated ROC AUC values were 0.981 and 0.964, respectively (Fig. 7). The domain with highest discriminatory power for NP-C in these age groups was the neurological domain, with ROC AUC values of 0.994 (4–16 years) and 0.873 (>16 years), respectively. The weakest individual domain was the visceral category. The discriminatory performance of the NP-C SI in infantile patients (<4 years of age) was poor, with ROC AUC value of 0.562 for the total RPS, with related poor performance in all three individual domains (visceral AUC = 0.597, neurological AUC = 0.615, and psychiatric AUC = 0.501).
Receiver operating characteristic (ROC) curves. Niemann-Pick disease type C (NP-C)-positive versus combined NP-C-suspected cases and controls by age. ROC curves that show higher sensitivity and specificity for NP-C-positive cases versus combined NP-C-suspected cases and controls in age groups 4–16 years (juvenile) and >16 years (adolescent) compared with infantile patients (<4 years). AUC area under the curve
Discussion
This study was carried out to further determine the association of manifestations and the discriminatory power of the SI in different age groups of patients with NP-C. Infantile patients displayed frequent manifestations of prolonged neonatal jaundice, splenomegaly, and delayed developmental milestones. This was as expected due to these signs being highly characteristic of NP-C in this age group (Vanier 2010). Infants <4 years of age also demonstrated fewer cross-domain manifestations compared with juvenile and adolescent patients, particularly the near absence of psychiatric manifestations. VSGP and gelastic cataplexia are the two strongest neurological indicators for NP-C according to the SI tool. These manifestations are usually present in low frequencies in infantile patients, if found at all. If VSGP is present, it may not be recognized, and the presence of gelastic cataplexia usually appears during the late infantile period (>3 years of age) (Patterson et al. 2012; Vanier 2010). Common neurological manifestations in infantile patients <4 years of age can include delays in developmental motor milestones, hypotonia, and language/speech delay (Vanier 2010; Patterson et al. 2012).
Whereas visceral manifestations appeared less frequently in adolescent patients (>16 years of age) with NP-C, neurological manifestations, including VSGP, dystonia, and dysarthria/dysphagia, showed an increased frequency compared with infant and juvenile patients. Epilepsy was the only manifestation to decrease in frequency between juvenile and adolescent patients. This could be an indicator of illness severity; mild, slowly progressive cases with less frequent or severe epilepsy may survive into adolescence and adulthood, whereas severe cases die during childhood.
Co-occurrence of manifestations both within and across domains greatly increases the suspicion level of NP-C, as evidenced by a high total RPS. Cross-domain manifestations in all three domains were experienced in higher proportions in patients >4 years of age than in infantile patients <4 years of age. Manifestations found across the visceral/psychiatric and visceral/neurological domains were the strongest indicators of NP-C, resulting in a higher point score within the NP-C SI tool than manifestations found across the neurological/psychiatric domains. The frequency of manifestations within the neurological/psychiatric domains increased in juvenile and adolescent patients, most noticeably, cognitive decline and ataxia.
The NP-C SI tool had strong discriminatory power (high ROC AUC) within and across the three separate domains for patients aged 4–16 years (juvenile) and > 16 years of age (adolescent) for NP-C-positive cases versus combined NP-C-suspected cases and controls, confirming the RPS 70 points suspicion threshold identified in the previously published study (Wijburg et al. 2012). The SI tool is less able to discriminate NP-C in infants <4 years of age. This could be due to an inability to detect specific neurological manifestations and the lack of development of psychiatric manifestations in this age group. The individual domain with the weakest ROC AUC value in this age group was the visceral domain. There is a need to develop an SI that is well suited to infantile patients. Protocol development is currently underway to collect more data for infantile patients with NP-C in order to develop a pediatric-specific SI tool.
Discrimination between specific (e.g., VSGP, cataplexy) and sensitive (e.g., ataxia, icterus prolonganus, cognitive decline) symptoms as strong indicators of NP-C, as well as the likelihood of any given symptom co-occurring with symptoms from other domains, was built into the NP-C SI tool during its initial construction when the individual RPS of each individual symptom was calculated and validated (Wijburg et al. 2012). Multivariable analysis of the SI tool to examine relationships between individual manifestations within categories was not possible due to insufficient data (occurrence of zero frequencies) for several binary predictors.
Other types of disease can present with similar visceral, neurological, and psychiatric manifestations to that of NP-C. Acid-sphingomyelinase-deficient Niemann-Pick disease, Sandhoff disease, and Gaucher disease type 3 can all present with visceral manifestations of hepatosplenomegaly/splenomegaly and neurological signs including ataxia and seizures. Patients with Huntington’s disease or progressive supranuclear gaze palsy can display neurological manifestations, including dystonia, VSGP, psychiatric symptoms, and cognitive decline (Patterson et al. 2012). However, suspicion for NP-C should be greatly increased when patients present clinical manifestations not only within, but more importantly across, multiple domains. Therefore, analysis of manifestations–association based on the SI tool concentrated on the complexity of manifestations across, rather than within, each domain. The NP-C SI tool is designed to allow physicians to objectively assess the likelihood of NP-C in undiagnosed patients. As such, it is not, and should not be, used as a differential diagnosis tool. It is possible that patients with other neurological disorders may score high on the NP-C SI (e.g. Alzheimer’s disease patients), but the precise diagnosis of these patients should be confirmed by further routine testing; it is also expected that incorrect diagnoses will become less common as physicians gain more experience using the SI tool.
Study strengths and limitations
A strength of the original published study, and therefore of these subanalyses, is that control cases were selected from the same outpatient clinics, where possible, as the NP-C-positive cases and suspected cases. This arguably increases the validity of the tool in routine clinical practice.
One notable limitation is the retrospective data being collected from a small group of NP-C-positive infantile patients (<4 years of age, n = 23). As NP-C is a rare disease, it is difficult for investigators to incorporate sufficient numbers of patients in studies, thereby making meaningful analysis a challenge. The current SI tool categorizes delayed developmental milestones in the ancillary category (allocated one point); however, this manifestation could be a stronger indicator of NP-C for this age group.
The SI tool can only be as good as the quality of patient data captured in the original study. This does not include patients who were never detected due to the atypical or nonspecific presentation of manifestations. As the disease is progressive, changing from visceral to neuropsychiatric in nature, the cross-sectional, retrospective nature of the data set makes it difficult to assess the usefulness of the SI in aiding early detection and predicting progression of NP-C in these patients. Further retrospective and future prospective, longitudinal studies may help to determine how useful the NP-C SI tool is in clinical practice and also to refine its use.
Conclusions
The NP-C SI tool is useful for providing information regarding association of manifestations within and across visceral, neurological, and psychiatric domains in infantile, juvenile, and adolescent patients with NP-C. The screening tool provides strong predictive power for suspicion of NP-C in patients >4 years of age but it is not as useful for infantile patients <4 years of age.
References
Chien YH, Peng SF, Yang CC, et al. (2013) Long-term efficacy of miglustat in paediatriC-patients with Niemann-Pick disease type C. J Inherit Metab Dis. 36:129–137
European Medicines Agency (2012) Summary of the European Public Assessment Report (EPAR) for Zavesca (miglustat). Retrieved May 18, 2012. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000435/WC500046724.pdf
Orphanet Report Series, Rare Diseases collection, November 2012, Number 1: Listed in alphabetical order of disease or group of diseases, http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf
Patterson MC, Hendriksz CJ, Walterfang M, Sedel F, Vanier MT, Wijburg F, NP-C Guidelines Working Group (2012) Recommendations for the diagnosis and management of Niemann-Pick disease type C: an update. Mol Genet Metab 106:330–344
Pineda M, Perez-Poyato MS, O’Callaghan M et al (2010) Clinical experience with miglustat therapy in pediatriC-patients with Niemann-Pick disease type C: a case series. Mol Genet Metab 99:358–366
Vanier MT (2010) Niemann-Pick disease type C. Orphanet J Rare Dis 5:16
Wijburg FA, Sedel F, Pineda M et al (2012) Development of a suspicion index to aid diagnosis of Niemann-Pick disease type C. Neurology 78:1560–1567
Wraith JE, Imrie J (2009) New therapies in the management of Niemann-Pick type C disease: clinical utility of miglustat. Ther Clin Risk Manag 5:877–887
Wraith JE, Baumgartner MR, Bembi B et al (2009) Recommendations on the diagnosis and management of Niemann-Pick disease type C. Mol Genet Metab 98:152–165
Wraith JE, Vecchio D, Jacklin E et al (2010) Miglustat in adult and juvenile patients with Niemann-Pick disease type C: long-term data from a clinical trial. Mol Genet Metab 99:351–357
Acknowledgments
Further statistical advice was provided by Professor Martin Schumacher of University Medical Center Freiburg, Freiburg, Germany. Editorial support during the preparation of this manuscript was provided by PHOCUS Services Ltd, a member of the Fishawack Group of Companies.
Study sponsorship and funding
The study was funded by a support grant from Actelion Pharmaceuticals Ltd, Allschwil, Switzerland. The authors confirm independence from the sponsor; the content of the article has not been influenced by the sponsor.
Competing interest
J.E. Wraith, F. Sedel, M. Pineda, F.A. Wijburg, C.J. Hendriksz, M. Fahey, M. Walterfang, and M.C. Patterson have all received consulting fees or honoraria from Actelion Pharmaceuticals Ltd.
S.A. Kolb and H. Chadha-Boreham are employees of Actelion Pharmaceuticals Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Olaf Bodamer
Professor James E. Wraith sadly passed away during the preparation of this manuscript. His co-authors would like to dedicate this paper to his memory.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Wraith, J.E., Sedel, F., Pineda, M. et al. Niemann-Pick type C Suspicion Index tool: analyses by age and association of manifestations. J Inherit Metab Dis 37, 93–101 (2014). https://doi.org/10.1007/s10545-013-9626-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-013-9626-y