Abstract
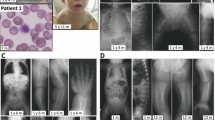
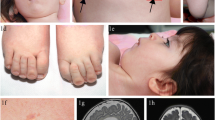
Recent years have seen great advances in our knowledge of congenital disorders of glycosylation (CDG), a clinically and biochemically heterogeneous group of genetic diseases caused by defects in the synthesis (CDG-I) or processing (CDG-II) of glycans that form glycoconjugates. This paper reports a new subtype of non-neurological CDG involving the impaired cytoplasmic biosynthesis of nucleotide sugars needed for glycan biosynthesis. A patient presented with muscle fatigue, elevated creatine kinase, growth hormone deficiency, and first branchial arch syndrome. These findings, together with the abnormal type II plasma transferrin isoform profile detected, was compatible with a CDG. Functional testing and clinical analyses suggested a deficiency in the interconversion of glucose-1-phosphate and glucose-6-phosphate catalyzed by phosphoglucomutase (PGM1), a defect previously described as glycogenosis type XIV (GSDXIV, MIM 612934). PGM1 activity in patient-derived fibroblasts was significantly reduced, as was the quantity of immunoreactive PGM1 protein (Western blot assays). Mutation analysis of PGM1 and subsequent functional analysis investigating transient expression of PGM1 in immortalized patient fibroblasts, followed by ex vivo splicing assays using minigenes, allowed the characterization of two novel pathogenic mutations: c.871G>A (p.Gly291Arg) and c.1144 + 3A>T. The latter represents a severe splicing mutation leading to the out-of-frame skipping of exon 7 and the formation of a truncated protein (p.Arg343fs). MALDI mass spectra of permethylated protein N-glycans from the patient’s serum suggested a marked hypoglycosylation defect. The present findings confirm that, in addition to a rare muscular glycolytic defect, PGM1 deficiency causes a non-neurological disorder of glycosylation.



Similar content being viewed by others
References
Azakir BA, Di Fulvio S, Kinter J, Sinnreich M (2012) Proteasomal inhibition restores biological function of mis-sense mutated dysferlin in patient-derived muscle cells. J Biol Chem 287:10344–10354
Bang YL, Nguyen TT, Trinh TT, Kim YJ, Song J, Song YH (2009) Functional analysis of mutations in UDP-galactose-4-epimerase (GALE) associated with galactosemia in Korean patients using mammalian GALE-null cells. FEBS J 276:1952–1961
Freeze HH, Sharma V (2010) Metabolic manipulation of glycosylation disorders in humans and animal models. Semin Cell Dev Biol 21:655–662
Guillard M, Morava E, van Delft FL, Hague R, Korner C, Adamowicz M, Wevers RA, Lefeber DJ (2011) Plasma N-glycan profiling by mass spectrometry for congenital disorders of glycosylation type II. Clin Chem 57:593–602
Jaeken J (2010) Congenital disorders of glycosylation. Ann N Y Acad Sci 1214:190–198
Lai K, Langley SD, Khwaja FW, Schmitt EW, Elsas LJ (2003) GALT deficiency causes UDP-hexose deficit in human galactosemic cells. Glycobiology 13:285–294
Lefeber DJ, Morava E, Jaeken J (2011) How to find and diagnose a CDG due to defective N-glycosylation. J Inherit Metab Dis 34:849–852
Mills PB, Mills K, Mian N, Winchester BG, Clayton PT (2003) Mass spectrometric analysis of glycans in elucidating the pathogenesis of CDG type IIx. J Inherit Metab Dis 26:119–134
Morava E, Lefeber D (2011) CDG - an update. J Inherit Metab Dis 34:847–848
Morelle W, Michalski JC (2007) Analysis of protein glycosylation by mass spectrometry. Nat Protoc 2:1585–1602
Perez-Cerda C, Clavero S, Perez B, Rodriguez-Pombo P, Desviat LR, Ugarte M (2003) Functional analysis of PCCB mutations causing propionic acidemia based on expression studies in deficient human skin fibroblasts. Biochim Biophys Acta 1638:43–49
Perez-Cerda C, Quelhas D, Vega AI, Ecay J, Vilarinho L, Ugarte M (2008) Screening using serum percentage of carbohydrate-deficient transferrin for congenital disorders of glycosylation in children with suspected metabolic disease. Clin Chem 54:93–100
Putt W, Ives JH, Hollyoake M, Hopkinson DA, Whitehouse DB, Edwards YH (1993) Phosphoglucomutase 1: a gene with two promoters and a duplicated first exon. Biochem J 296(Pt 2):417–422
Rincon A, Aguado C, Desviat LR, Sanchez-Alcudia R, Ugarte M, Perez B (2007) Propionic and methylmalonic acidemia: antisense therapeutics for intronic variations causing aberrantly spliced messenger RNA. Am J Hum Genet 81:1262–1270
Stojkovic T, Vissing J, Petit F, Piraud M, Orngreen MC, Andersen G, Claeys KG, Wary C, Hogrel JY, Laforet P (2009) Muscle glycogenosis due to phosphoglucomutase 1 deficiency. N Engl J Med 361:425–427
Sturiale L, Barone R, Fiumara A, Perez M, Zaffanello M, Sorge G, Pavone L, Tortorelli S, O’Brien JF, Jaeken J, Garozzo D (2005) Hypoglycosylation with increased fucosylation and branching of serum transferrin N-glycans in untreated galactosemia. Glycobiology 15:1268–1276
Van Schaftingen E, Jaeken J (1995) Phosphomannomutase deficiency is a cause of carbohydrate-deficient glycoprotein syndrome type I. FEBS Lett 377:318–320
Wopereis S, Grunewald S, Morava E, Penzien JM, Briones P, Garcia-Silva MT, Demacker PN, Huijben KM, Wevers RA (2003) Apolipoprotein C-III isofocusing in the diagnosis of genetic defects in O-glycan biosynthesis. Clin Chem 49:1839–1845
Acknowledgements
This work was funded by grants from the Spanish Ministerio de Ciencia e Innovación (PI10/00455 to BP and PI11/01254 to CPC). An institutional grant from the Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa is gratefully acknowledged. Authors also thank Mª Teresa Alonso from Servicio Interdepartamental de Investigación (SIDI-UAM) for glycan analysis by MALDI-TOF-mass spectrometry.
Details of funding
The authors confirm independence from the sponsors, and the content of the article has not been influenced by the sponsors.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Eva Morava
Rights and permissions
About this article
Cite this article
Pérez, B., Medrano, C., Ecay, M.J. et al. A novel congenital disorder of glycosylation type without central nervous system involvement caused by mutations in the phosphoglucomutase 1 gene. J Inherit Metab Dis 36, 535–542 (2013). https://doi.org/10.1007/s10545-012-9525-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-012-9525-7