Abstract
Background & Aims
Wilson disease (WD) is an inherited disorder of copper disposition caused by an ATP7B transporter gene mutation, leading to copper accumulation in predisposed tissues. In addition to a genetic predisposition, other factors are likely to contribute to its clinical manifestation. The aim of the study was to assess whether oxidative stress affects the phenotypic manifestation of WD.
Methods
In 56 patients with WD (29 men; 26 with the hepatic form, 22 with the neurologic form, and eight asymptomatic; mean age 38.5 ± 12 years), total serum antioxidant capacity (TAC) and inflammatory parameters (hs-CRP, IL-1β, IL-2, IL-6, IL-10, and TNF-α) were analyzed and related to the clinical manifestation, and mutations of the ATP7B gene. The control group for the TAC and inflammatory parameters consisted of 50 age- and gender-matched healthy individuals.
Results
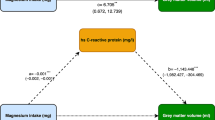
WD patients had a significantly lower TAC (p < 0.00001), lower IL-10 levels (p = 0.039), as well as both higher IL-1β (p = 0.019) and IL-6 (p = 0.005) levels compared to the control subjects. TNF-α, hs-CRP, and IL-2 did not differ from the controls. Patients with the neurological form of WD had a significantly lower TAC than those with the hepatic form (p < 0.001). In addition, the lower TAC was associated with the severity of the neurological symptoms (p = 0.02). No relationship between the inflammatory parameters and clinical symptoms was found.
Conclusions
Data from our study suggest that the increased oxidative stress contributes significantly to the clinical manifestation of WD; as a lower TAC is associated with the neurological symptoms in WD patients.



Similar content being viewed by others
References
Angulo P (2002) Nonalcoholic fatty liver disease. N Engl J Med 346:1221–1231
Borjigin J, Payne AS, Deng J et al. (1999) A novel pineal night-specific ATPase encoded by the Wilson disease gene. J Neurosci 19:1018–1026
Britton RS (1996) Metal-induced hepatotoxicity. Semin Liver Dis 16:3–12
Bruha R, Marecek Z, Pospisilova L et al. (2011) Long-term follow-up of Wilson Disease: natural history, treatment, mutations analysis and phenotypic correlation. Liver Int 31:83–91
Bull AI, Thomas GR, Rommens JM, Forbes JR, Cox DW (1993) The Wilson disease gene is a putative copper transporting P-type ATPase similar to Menkes gene. Nat Genet 5:327–337
Evenson MA (1988) Measurement of copper in biological samples by flame or electrothermal atomic absorption spectrometry. Method Enzymol 158:351–357
Ferenci P (2005) Wilson’s disease. Clin Gastroenterol Hepatol 3:726–733
Ferenci P, Caca K, Loudianos G et al. (2003) Diagnosis and phenotypic classification of Wilson disease. Liver Int 23:139–142
Ferns GA, Lamb DJ, Taylor A (1997) The possible role of copper ions in atherogenesis: the Blue Janus. Atherosclerosis 135:139–152
Fryer MJ (2009) Potential of vitamin E as an antioxidant adjunct in Wilson’s disease. Med Hypotheses 73:1029–1030
Gu M, Cooper JM, Butler P et al. (2000) Oxidative-phosphorylation defects in liver of patients with Wilson’s disease. Lancet 356:469–474
Iuliano L, Piccheri C, Coppola I, Praticò D, Micheletta F, Violi F (2000) Fluorescence quenching of dipyridamole associated to peroxyl radical scavenging: a versatile probe to measure the chain breaking antioxidant activity of biomolecules. Biochim Biophys Acta 1474:177–182
Jimenez I, Aracena P, Letelier ME, Navarro P, Speisky H (2002) Chronic exposure to HepG2 cells to excess copper results in depletion of glutathione and induction of metallothionein. Toxicol In Vitro 16:167–175
Maier-Dobersberger T, Ferenci P, Polli C et al. (1997) Detection of the His1069Gln mutation in Wilson disease by rapid polymerase chain reaction. Ann Intern Med 127:21–26
Merle U, Schaefer M, Ferenci P, Stremmel W (2007) Clinical presentation, diagnosis and long-term outcome of Wilson´s disease: a cohort study. Gut 56:115–120
Nagasaka H, Inoue I, Inui A et al. (2006) Relationship between oxidative stress and antioxidant systems in the liver of patients with Wilson disease: hepatic manifestation in Wilson disease as a consequence of augmented oxidative stress. Pediatr Res 60:472–477
Nagasaka H, Takayanagi M, Tsukahara H (2009) Children’s toxicology from bench to bed - Liver Injury (3): Oxidative stress and anti-oxidant systems in liver of patients with Wilson disease. J Toxicol Sci 34:229–236
Nevsimalova S, Buskova J, Bruha R et al. (2011) Sleep disorders in Wilson’s disease. Eur J Neurol 18:184–190
Nicastro E, Loudianos G, Zancan L et al. (2009) Genotype-phenotype correlation in Italian children with Wilson’s disease. J Hepatol 50:555–561
Ogihara H, Ogihara T, Miki M, Yasuda H, Mino M (1995) Plasma copper and antioxidant status in Wilson’s disease. Pediatr Res 37:219–226
Ojeda CB, Rojas FS, Pavon JMC (1995) Recent developments in derivative ultraviolet/visible absorption spectrophotometery. Talanta 42:1195–1214
Roberts EA, Schilsky ML (2008) Diagnosis and treatment of Wilson disease: An update. Hepatology 47:2089–2111
Rodo M, Czlonkowska A, Pulawska M, Swiderska M, Tarnacka B, Wehr H (2000) The level of serum lipids, vitamin E and low density lipoprotein oxidation in Wilson’s disease patients. Eur J Neurol 7:491–494
Scheinberg IH (1981) Wilson’s disease. J Rheumatol Suppl 7:90–93
Schiefermeier M, Kollegger H, Madl C et al. (2000) The impact of apolipoprotein E genotypes on age at onset of symptom and phenotypic expression in Wilson’s disease. Brain 123:585–590
Sies H (1993) Strategies of antioxidant defense. Eur J Biochem 215:213–219
Simon I, Schaefer M, Reichert J, Stremmel W (2008) Analysis of the human Atox 1 homologue in Wilson patients. World J Gastroenterol 14:2383–2387
Sinha S, Christopher R, Arunodaya GR et al. (2005) Is low serum tocopherol in Wilson’s disease a significant symptom? J Neurol Sci 228:121–123
Sokol RJ, Twedt D, McKim JM et al. (1994) Oxidant injury to hepatic mitochondria in patients with Wilson’s disease and Bedlington terriers with copper toxicosis. Gastroenterology 107:1788–1798
Sokol RJ, McKim JM Jr, Devereaux MW (1996) Alpha-tocopherol ameliorates oxidant injury in isolated copper-overloaded rat hepatocytes. Ped Res 39:259–263
Spisni E, Valerii MC, Manerba M et al. (2009) Effect of copper on extracellular levels of key pro-inflammatory molecules in hypothalamic GN11 and primary neurons. Neurotoxicology 30:605–612
Stapelbroek JM, Bollen CW, Ploos van Amstel JK et al. (2004) The H1069Q mutation in ATP7B is associated with late and neurologic presentation in Wilson disease: results of a meta-analysis. J Hepatol 41:758–763
Sternlieb I, Scheinberg IH (1968) Prevention of Wilson’s disease in asymptomatic patients. N Engl J Med 278:352–359
Tanzi RE, Petrukhin K, Chernov I et al. (1993) The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet 5:344–350
Thomas GR, Forbes JR, Roberts EA, Walshe JM, Cox DW (1995) The Wilson disease gene spectrum of mutations and their consequences. Nat Genet 9:210–217
Videla LA, Fernandez V, Tapia G, Varela P (2003) Oxidative stress-mediated hepatotoxicity of iron and copper: Role of Kupfer cells. Biometals 16:103–111
von Herbay A, de Groot H, Hegi U, Stremmel W, Strohmeyer G, Sies H (1994) Low vitamin E content in plasma of patients with alcoholic liver disease, hemochromatosis and Wilson’s disease. J Hepatol 20:41–6
Vrabelova S, Letocha O, Borsky M, Kozak L (2005) Mutation analysis of the ATP7B gene and genotype/phenotype correlation in 227 patients with Wilson disease. Mol Genet Metab 86:277–285
Walter U, Krolikowski K, Tarnacka B, Benecke R, Czlonkowska A, Dressler D (2005) Sonographic detection of basal ganglia lesions in aswymptomatic and symptomatic Wilson disease. Neurology 64:1726–1732
Weiss KH, Lozoya JC, Tuma S et al. (2008) Copper-induced translocation of the Wilson disease protein ATP7B independent of Murr1/COMMD1 and Rab7. Am J Pathol 173:1783–1794
Weiss KH, Runz H, Noe B et al. (2010) Genetic analysis of BIRC4/XIAP as a putative modifier gene of Wilson disease. J Inherit Met Dis doi:10.1007/s10545-010-9123-5
Wright LM, Huster D, Lutsenko S, Wrba F, Ferenci P, Fimmel CJ (2009) Hepatocyte GP73 expression in Wilson disease. J Hepatol 51:557–564
Zischka H, Lichtmannegger J, Schmitt S et al. (2011) Liver mitochondrial membrane crosslinking and destruction in a rat model of Wilson dinase. J Clin Invest 121:1508–1518
Acknowledgment
The authors would like to thank Mgr. Skibova from IKEM Prague for her help with the statistical analysis.
Grant support
The study was supported by grant IGA MZ CR No. NT 12290/4 and NT 11247/4 provided by the IGA, Czech Ministry of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Moacir Wajner
Competing interests: None declared.
Rights and permissions
About this article
Cite this article
Bruha, R., Vitek, L., Marecek, Z. et al. Decreased serum antioxidant capacity in patients with Wilson disease is associated with neurological symptoms. J Inherit Metab Dis 35, 541–548 (2012). https://doi.org/10.1007/s10545-011-9422-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-011-9422-5