Abstract
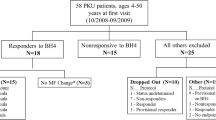
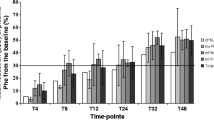
The impact of tetrahydrobiopterin (BH4) treatment on phenylalanine tolerance, medical-food consumption, and nutrition status in patients with phenylketonuria (PKU) was investigated. Six children (5–12 years) with well-controlled PKU, responding to a BH4 dose of 20 mg/kg per day, were assessed for 24 months. Mean dietary phenylalanine tolerance increased from 421 ± 128 to 1470 ± 455 mg/day. Height Z scores significantly improved from 0.25 ± 0.99 at baseline to 0.53 ± 1.16 at 24 months (p for trend < 0.001). Patients tolerated more phenylalanine and more intact protein and required less medical food (protein supplement). Improved linear growth and nutrition status was seen over the course of the 24-month follow-up. Due to the variation in phenylalanine tolerance, dietary recommendations should be tailored to the patient’s individual requirements.



Similar content being viewed by others
References
Bilginsoy C, Waitzman N, Leonard CO, Ernst SL (2005) Living with phenylketonuria: perspectives of patients and their families. J Inherit Metab Dis 28:639–649
Finkelson L, Bailey I, Waisbren SE (2001) Short report: PKU adults and their return to diet: predicting diet continuation and maintenance. J Inherit Metab Dis 24:515–516
Frank N, Fitzgerald R, Legge M (2007) Phenylketonuria—the lived experience. N Z Med J 120:1262
Institute of Medicine of the National Academies, Food and Nutrition Board (2002) Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Retrieved 15 May 2009 from: http://www.iom.edu/CMS/3788/4576/4340.aspx
Lee P, Treacy EP, Crombez E et al (2008) Safety and efficacy of 22 weeks of treatment with sapropterin dihydrochloride in patients with phenylketonuria. Am J Med Genet A 146A:2851–2859
Levy HL, Milanowski A, Chakrapani A et al (2007) Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study. Lancet 370:504–510
MacDonald A (2000) Diet and compliance in phenylketonuria. Eur J Ped 159(2):S136
Mayo Medical Laboratories online catalogue. Retrieved 17 Apr 2009 from http://www.mayomedicallaboratories.com/test-catalog/
Medical Research Council (1993) Recommendations on the dietary management of phenylketonuria. Report of Medical Research Council Working Party on Phenylketonuria. Arch Dis Child 68:426–427
National Academy of Sciences. Institute of Medicine. Food and Nutrition Board (2005) Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Retrieved 30 Jan 2009 from: http://fnic.nal.usda.gov/nal_display/index.php?info_center=4&tax_level=4&tax_subject=256&topic_id=1342&level3_id=5141&level4_id=10588
National Health and Nutrition Examination Survey, Panel on Macronutrients (2002) Retrieved 30 Jan 2009 from: http://www.cdc.gov/nchs/nhanes.htm
National Institutes of Health Consensus Development Panel (2001) National Institutes of Health Consensus Development Conference Statement: phenylketonuria: screening and management, October 16–18, 2000. Pediatrics 108(4):972–982
Singh RH, Dembure P, Eisensmith RC, Guerrero NV, Sullivan K, Elsas LJ (1999) Pretreatment serum phenylalanine levels as a predictor of the severity of the mutation in the phenylalanine hydroxylase gene and phenylalanine tolerance in patients with phenylketonuria. J Am Diet Assoc 99(9):A15
Singh RH, Jurecki E, Rohr R (2008) Recommendations for personalized dietary adjustments based on patient response to tetrahydrobiopterin (BH4) in phenylketonuria. Top Clin Nutr 23(2):149–157
Steinfeld R, Kohlschütter A, Ullrich K, Lukacs L (2004) Efficiency of long-term tetrahydrobiopterin monotherapy in phenylketonuria. J Inherit Metab Dis 27:449–453
Trefz FK, Scheible KD, Frauendienst-Egger G, Korall H, Blau (2005) Long-term treatment of patients with mild and classical phenylketonuria by tetrahydrobiopterin. Mol Genet Metab 86:S75–S80
Walter JH, White FJ, Hall SK et al (2002) How practical are recommendations for dietary control in phenylketonuria? The Lancet 360:55–57
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: John H. Walter
Competing interest: None declared.
Rights and permissions
About this article
Cite this article
Singh, R.H., Quirk, M.E., Douglas, T.D. et al. BH4 therapy impacts the nutrition status and intake in children with phenylketonuria: 2-year follow-up. J Inherit Metab Dis 33, 689–695 (2010). https://doi.org/10.1007/s10545-010-9224-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-010-9224-1