Abstract
The heterogeneous group of 3-methylglutaconic aciduria (3-MGA-uria) syndromes includes several inborn errors of metabolism biochemically characterized by increased urinary excretion of 3-methylglutaconic acid. Five distinct types have been recognized: 3-methylglutaconic aciduria type I is an inborn error of leucine catabolism; the additional four types all affect mitochondrial function through different pathomechanisms. We provide an overview of the expanding clinical spectrum of the 3-MGA-uria types and provide the newest insights into the underlying pathomechanisms. A diagnostic approach to the patient with 3-MGA-uria is presented, and we search for the connection between urinary 3-MGA excretion and mitochondrial dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Branched-chain organic acid 3-methylglutaconic acid: the biochemical basis
The branched-chain organic acid 3-methylglutaconic acid (3-MGA) is an intermediate of the mitochondrial leucine catabolism. Figure 1 shows the metabolic pathway of leucine; 3-MGA, 3-methylglutaric acid (3-MG), and 3-hydroxyisovaleric acid (3-HIVA) accumulate when the conversion of 3-methylglutaconyl-coenzyme A (CoA) to 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) by the enzyme 3-methylglutaconyl-CoA hydratase (3-MGH, EC 4.2.1.18) is disturbed. This is the underlying cause in 3-MGA-uria type I. As we show in detail upon describing the different subtypes of 3-MGA-uria, there is no evidence that the 3-MGA-uria types II–V are caused by a disturbed leucine catabolism. Notably, subtypes II–V affect mitochondrial function through different pathomechanisms. But how can mitochondrial dysfunction lead to elevated urinary excretion of 3-MGA?
Leucine catabolism and possible shunts to cholesterol biosynthesis. 1 Transaminase, 2 branched-chain 2-oxo-acid dehydrogenase, 3 isovaleryl-CoA dehydrogenase, 4 3-methylcrotonyl-CoA carboxylase, 5 3-methylglutaconyl-CoA-hydratase, 6 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) lyase, 7 HMG-CoA-synthase, 8 HMG-CoA-reductase. PPi pyrophosphate
In the urine of healthy individuals, 3-MGA is found only in traces (< 20 mmol/mol creatinine). In patients with 3-MGA-uria, concentrations can (intermittently) rise above 1,000 mmol/mol creatinine. The urinary excretion of 3-MGA is generally higher in type I than in the other types (Elpeleg et al. 1994; Christodoulou et al. 1994; Cantlay et al. 1999; Schmidt et al. 2004). Patients with 3-MGA-uria type I excrete even higher amounts of urinary 3-MGA after a leucine-rich, or in general, a protein-rich meal (Duran et al. 1982; Ensenauer et al. 2000). This is not the case in patients with the other types of 3-MGA-uria, which emphasizes that the excreted 3-MGA does not originate from leucine degradation (Barth syndrome: Kelley et al. 1991; Christodoulou et al. 1994; type IV: Chitayat et al. 1992; Wortmann et al. unpublished data). Interestingly, in these patients, the excretion can be highly variable or intermittently absent, even within 24 h, seemingly unrelated to the clinical course or severity of the metabolic derangement (Elpeleg et al. 1994; Cantlay et al. 1999; Schmidt et al. 2004; Christodoulou et al. 1994; Wortmann et al. 2009).
Patients with 3-MGA-uria type II (Barth syndrome) have both increased 3-MGA and low cholesterol levels. Therefore, it was speculated that some of the idiopathic syndromes with 3-MGA-uria may be caused by defects of sterol or isoprenoid metabolism causing overflow of mevalonate carbon through the so-called mevalonate shunt (Fig. 1; Kelley et al. 1991; Kelley and Kratz 1995). In this shunt, dimethylallyl pyrophosphate is dephosphorylated in two steps to the free alcohol, oxidized to 3-methylcrotonic acid, and then activated with CoA to form 3-methylcrotonyl-CoA, the precursor of 3-MGA-CoA in the regular leucine catabolic pathway (Edmond and Popjak 1974). Other links between the two pathways at the level of higher-order isoprenoids, such as geraniol and farnesol, have been described (Fig. 1; Edmond and Popjak 1974; (Schroepfer 1981). Also, a direct shunt between mevalonate and 3-methylglutaconyl-CoA was hypothesized (Fig. 1; Edmond and Popjak 1974). This is the only “mevalonate shunt” per se (Walsh et al. 1999). It seems that the shunt is more active in tissue of ectodermal origin (e.g., skin, placental tissue) than in tissue of mesodermal origin (Edmond and Popjak 1974). Physiologic evidence that the mevalonate shunt or a related shunt is significant in humans, at least in renal tissue, has been provided (Hughes-Fulford et al. 1986). More data are available for rats, in which the liver is the main organ of mevalonate shunting (Weinstock et al. 1984). Investigations in 35 patients with Smith-Lemli-Opitz syndrome (SLO, MIM 27400) showed a weak inverse correlation between low plasma cholesterol, its elevated precursor 7-dehydrocholesterol, and elevated plasma 3-MGA (Kelley and Kratz 1995). SLO patients with very low plasma cholesterol (< 200 μg/ml; seven of 35 patients) generally had plasma 3-MGA levels above the + 2 standard deviation (SD) range for age (five of seven patients; range 400–5,000 nmol/l). The rise in cholesterol precursors (isoprenoids), which cannot be metabolized to cholesterol in patients with SLO, leads to overflow via the mevalonate shunt and a consequential increase in 3-MGA (Fig. 1). The authors also hypothesized another mechanism. Low plasma cholesterol can induce HMG-CoA synthase, leading to increased HMG-CoA levels and an increased flux through the cholesterol biosynthesis pathway (Goldstein and Brown 1990). The HMG-CoA is then dehydrated to 3-MGA through 3-MGH (Fig. 1; Kelley and Kratz 1995). The urine of some patients also contained high amounts of 3-MGA but no increased 3-HIVA levels, which is characteristic for 3-MGA-uria type I, suggesting that the 3-MGA does not originate from leucine catabolism. Still, the described shunt does not explain how mitochondrial dysfunction relates to excessive 3-MGA excretion. Several enzymes involved in leucine degradation as well as sterol biosynthesis are nicotinamide adenine dinucleotide phosphate (NADP)–NADP, reduced (NADPH) dependent. One could hypothesize that oxidative phosphorylation system (OXPHOS) dysfunction influences NADP-NADPH-dependent enzymes, such as the 3-MGA-hydratase by a disturbed NADP/NADPH ratio. However, excretion seems unrelated to clinical severity or disease course. At the moment, 3-MGA is a biochemical marker for mitochondrial dysfunction of still unknown origin.
Measuring 3-MGA by gas chromatography/mass spectrometry (GC-MS) and one-dimensional [1H]-NMR spectroscopy
As part of the routine metabolic screening in our lab, urinary organic acid analysis is performed by gas chromatography/mass spectrometry (GC–MS) after extraction of the urine sample with ethyl acetate and derivatization with N,N-bis(trimethylsilyl)trifluoroacetamide containing 1% trimethylchlorosilane. The concentration of 3-MGA is quantified by comparing the signals obtained with calibration curves of the pure compound, using a CP-Sil 8 CB column (Varian, Middelburg, The Netherlands) on a high-performance (HP) 6890 Gas Chromatograph (Agilent, Amstelveen, The Netherlands). For research purposes, we additionally perform one-dimensional [1H]-nuclear magnetic resonance (NMR) spectroscopy of different body fluids (Fig. 2; Engelke et al. 2006). Body fluid samples are measured at 500 MHz on a Bruker DRX 500 spectrometer with a triple-resonance inverse (TXI) 1H {15N, 13C} probe head equipped with X,Y,Z gradient coils. 1H spectra are acquired as 128 transients in 32-K data points with a spectral width of 6,002 Hz. The water (H2O) resonance is presaturated by single-frequency irradiation during a relaxation delay of 10 s; a pulse width of 7 μs is used (corresponding to a 90° excitation pulse).
3-MGA-uria type I (MIM 250950)
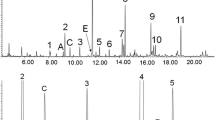
For a long time, 3-MGA-uria type I was thought to be a classic organic aciduria. It is a rare autosomal recessive disorder of leucine catabolism characterized by markedly increased urinary excretion of 3-MGA and mildly elevated urinary 3-MG and 3-HIVA. The underlying cause of 3-MGA-uria type I is deficiency of 3-MGH, the enzyme that catalyzes the fifth step of leucine catabolism, the conversion of 3-methylglutaconyl-CoA to HMG-CoA (Fig. 1). Murine 3-MGH is highly expressed in kidney, skeletal muscle, heart, and brain and was shown to be located in the mitochondria (Brennan et al. 1999). The activity of 3-MGH can be determined in fibroblasts or lymphocytes by use of an overall enzyme assay measuring three steps of leucine degradation, from 3-methylcrotonyl-CoA to acetoacetate (Narisawa et al. 1986; IJlst et al. 2002). 3-MGH is encoded by the AUH gene, which was mapped to chromosome 9 and encompasses ten exons encoding for a protein with 339 amino acids (Ijlst et al. 2002; Ly et al. 2003). The ratio of cis and trans isoforms of 3-MGA in urine of 3-MGA-uria type I patients is 2:1, whereas in cerebrospinal fluid (CSF), only the cis isoform is detectable (Engelke et al. 2006). In the other 3-MGA-uria types, the urinary cis:trans ratio is approximately 1:1 [Fig. 2; repetitive measurements in patients with 3-MGA-uria type I (n = 5), II (n = 5), III (n = 6), and IV (n > 80); (Wortmann et al. 2009)].
[1H]-nuclear magnetic resonance (NMR) spectra of patients with 3-methylglutaconic aciduria (3-MGA-uria) types I and IV. One-dimensional [1H]-NMR spectra (500 MHz) of urine measured at pH 2.5. The region between 2.2. and 1.9 ppm is shown. a 2:1 cis:trans ratio in the urine of a patient with 3-MGA-uria type I. b 1:1 cis:trans ratio in the urine of a patient with 3-MGA-uria type IV
It is known that 3-methylcrotonyl-CoA carboxylase specifically forms the trans form of 3-methylglutaconyl-CoA (Lynen et al. 1961). The metabolic origin of cis-3-methylglutaconyl-CoA remains as yet unknown. Spontaneous cis/trans interconversion may play a role. A brain-specific isoform of 3-MGH or an enzyme converting the trans form into the cis form, thus taking care of local production of the cis form in the brain, may be an explanation (Engelke et al. 2006).
3-MGA-uria was thought to present in childhood with nonspecific symptoms such as mental retardation or seizures. It was even speculated to be a nondisease (Gibson et al. 1998). Recently, we reported that it is, in fact, a slowly progressive leukoencephalopathy clinically presenting in adulthood (Wortmann et al. 2010). Function-abolishing mutations were reported in seven children with various nonspecific symptoms, such as mental retardation, seizures, hepatopathy (Ijlst et al. 2002; Ly et al. 2003; Illsinger et al. 2004; Matsumori et al. 2005). Recently, our group reported the biochemical details of the first patient with an adult onset of the disease, a Dutch woman (Engelke et al. 2006; Wortmann et al. 2010). She presented with a progressive bilateral visual decline and optic atrophy at the age of 35 years. Over the following 16 years, dysarthria and mild limb ataxia with severe gait ataxia were observed. One additional late-presenting patient was reported with dementia and spasticity by a Japanese group (Eriguchi et al. 2006). Both had a slowly progressive leukoencephalopathy. The same clinical signs and symptoms and disease course was observed in a third adult patient who came to our attention (Wortmann et al. 2010). This British man first presented at the age of 30 years with mild cerebellar ataxia and slowly worsened over 29 years, showing spastic paraparesis, nystagmus, and dementia. Magnetic resonance imaging (MRI) of our adult patients showed extensive and diffusively distributed white-matter lesions restricted to the supratentorial region not affecting the cerebellum or the corpus callosum [for MRI/MR spectrometry (MRS), see Engelke et al. (2006)]. The Japanese patient also showed cerebellar involvement (Eriguchi et al. 2006). This led us to perform an MRI in a pediatric 3-MGA-uria type I patient who was detected upon metabolic screening for recurrent febrile seizures (in total, 15 up to age 7 years) at the age of 4 years (Illsinger et al. 2004). He is 10 years old at this writing and has developed completely normally. His MRI showed mild signal abnormalities in deep frontal white matter with sparing of the U fibers. We propose that these abnormalities represent the earliest stages of the slowly progressive neurodegenerative disorder mainly affecting the white matter observed in the adult patients. Metabolite accumulation may contribute to the clinical signs and symptoms of this disease. A toxic effect of 3-MGA on the cerebral cortex has been demonstrated in rats (Leipnitz et al. 2008). There have also been speculations about the neurotoxicity of 3-HIVA (Duran et al. 1993). The obvious accumulation of 3-HIVA in CSF and brain observed by MRS in the Dutch woman may be indicative for a central role of 3-HIVA accumulation in the natural course of brain damage in this disease (Engelke et al. 2006). As in other organic acidurias, accumulation of toxic metabolites may give rise to slow-onset excitotoxicity with cellular dysfunction and eventually cell death. If the natural-course scenario that we propose can be confirmed in a larger series of patients, leucine-restricted diet as a therapeutic approach from childhood onward must be reconsidered.
3-MGA-uria type II (Barth syndrome, MIM 302060)
The 3-MGA-uria type II or Barth syndrome is an X-linked recessive cardiomyopathy with (cyclic) neutropenia, skeletal myopathy, and mitochondrial respiratory chain dysfunction first described in a large Dutch family some 30 years ago (Barth et al. 1981, 1983). Ten years later, the 3-MGA-uria and decreased plasma cholesterol were added as consistent disease features (Kelley et al. 1991). Sudden unexpected death in early life has been reported (Yen et al. 2008). The progression of cardiomyopathy (CM) is variable, sometimes slowly improving over the years, but mostly progressive and ending up at a point where heart transplantation is the only treatment option (Christodoulou et al. 1994; Adwani et al. 1997; Mangat et al. 2007). Cyclic neutropenia, ranging from mild to severe, is frequently seen; and fatal bacterial infections can occur in the neonatal period. Neutropenia and CM can develop simultaneously or in isolation. Onset ranges between birth and 49 years and peaks around puberty (Barth et al. 2004). Chronic aphthous ulceration due to Candida infections is a common sequela. Treatment with granulocyte colony-stimulating factor (G-CSF) seems to be successful and safe (Dale et al. 2006). Most patients show a degree of growth deficiency with height following the −2 SD percentile (Barth et al. 2004). There is evidence that patients share distinct facial features (tall and broad forehead, round face with prominent chin and full cheeks, large ears, and deep-set eyes), which are most evident in infancy (Hastings et al. 2009). In early studies, normal mental functioning and intelligence is reported. Recent studies suggest a higher incidence of cognitive difficulties with regard to mathematics, visual spatial tasks, and short-term memory. Language ability is spared. In combination with the excess fatigue often seen in these patients, this should be given special attention (Mazzocco et al. 2007).
The excretion of 3-MGA in urine can be highly variable, even within 24 h, and is often intermittent (Christodoulou et al. 1994; Cantlay et al. 1999). The 3-MGA-uria is seemingly unrelated to the clinical course or severity of metabolic derangement. Even patients without 3-MGA-uria have been described (Schmidt et al. 2004). Other characteristic findings in the urine are increased levels of 3-MG and 2-ethylhydracrylic acid, the latter a consequence of the isoleucine breakdown. Neither prolonged fasting nor leucin loading tests leads to changes in 3-MGA excretion, suggesting an alternative source of 3-MGA in affected patients (Kelley et al. 1991; Christodoulou et al. 1994). Moderately decreased plasma total cholesterol, mostly belonging to the low-density lipoprotein (LDL) pool, is a consistent finding (Kelley et al. 1991). This led to the hypothesis that 3-MGA results from overflow via the mevalonate shunt (Fig. 1; Kelley et al. 1991; Kelley and Kratz 1995). Cells from patients with Barth syndrome show a characteristic abnormal cardiolipin profile, which is the basis for the diagnosis (for review, see Houtkooper et al. 2009). Total cardiolipin levels are lower, especially of the tetralineoyl subclasses, and the acyl chain composition is shifted toward less unsaturated species with markedly elevated monolysocardiolipin (Valianpour et al. 2002). Cardiolipin is primarily found in the inner mitochondrial membrane and to a lesser extent in the outer mitochondrial membrane. Several proteins of the respiratory chain have been reported to bind to cardiolipin or require cardiolipin for optimal activity (as reviewed in Houtkooper and Vaz 2008). Furthermore, cardiolipin is reported to function in the stabilization of the individual respiratory chain complexes in a larger so-called supercomplex, enabling efficient channelling of electrons through the complexes. Barth syndrome is caused by mutations in the TAZ gene located at Xq28, encoding the protein tafazzin, named after the masochistic comic character from an Italian TV sport show (Bione et al. 1996). The function of this mitochondrial cardiolipin transacylase and its different splice variants (129–292 amino acids long) in the remodelling of cardiolipin remains elusive. A role in apoptosis has been suggested, but how this causes CM and neutropenia is unknown (Houtkooper and Vaz 2008).
3-MGA-uria type III (Costeff syndrome, MIM 258501)
The 3-MGA-uria type III, or Costeff syndrome, is an autosomal recessive disorder with infantile bilateral optic atrophy, extrapyramidal signs, spasticity, ataxia, dysarthria, and cognitive deficit in decreasing order of frequency. It was first described in 19 Israeli patients in 1989 (Costeff et al. 1989). The excretion of 3-MGA and 3-MG is, as in the other 3-MGA-uria types, quite variable (Elpeleg et al. 1994). All patients of Iraqi Jewish origin are homozygous for a splice site founder mutation in OPA3 (mapped to 19q13.2-q13.3; Anikster et al. 2001). Several patients have been reported since then, almost exclusively of Iraqi Jewish origin, with the exception of one Turkish Kurdish and one Indian patient harboring different mutations (Kleta et al. 2002; Neas et al. 2005; Ho et al. 2008). Two other OPA3 mutations result in a rare dominant disorder (ADOAC; MIM 165300) involving optic atrophy, cataracts, and extrapyramidal signs without 3-MGA-uria (Reynier et al. 2004; Verny et al. 2005). The OPA3 gene was first thought to consist of two exons; recently, it was proven to compromise three exons, resulting in two gene transcripts—OPA3A and OPA3B (Anikster et al. 2001; Huizing et al. 2010). Both transcripts contain exon 1, which is spliced to exon 2 in OPA3A (179 amino acids) and exon 3 in OPA3B (180 amino acids). OPA3A is expressed and conserved from fungi to primates, whereas OPA3B is uniquely found in mammals. In contrast to OPA3A, OPA3B is not identified in the proteomic database and is considerably less frequently expressed in wild-type cells (Huizing et al. 2010). Recently, the prediction of a mitochondrial localization of the OPA3A protein could be confirmed. It is an integral protein of the mitochondrial outer membrane. The authors also reported an integral role for OPA3A in mitochondrial fission and apoptosis (Ryu et al. 2010). Very recently, a zebrafish model of Costeff syndrome has been described. Herein mitochondrial OPA3 is shown to protect the electron transport chain against inhibitory compounds (Pei et al. 2010).
3-MGA-uria type IV (MIM 250951)
Although about 100 patients with 3-MGA-uria not being classified as type I, II, III, or V have been described, OMIM refers to only one case report from 1992 for 3-MGA-uria type IV. This was a young man with severe psychomotor retardation, poor growth, subvalvular aortic stenosis, and CM. He later developed seizures, spasticity, and sensorineural hearing loss (Chitayat et al. 1992). Since then, the spectrum has expanded rapidly. The underlying etiology has not been elucidated as yet but is certainly heterogeneous (Gunay-Aygun 2005).
The majority of patients described so far presented with CM (Holme et al. 1992; Ibel et al. 1993; Besley et al. 1995; Ruesch et al. 1996; De Kremer et al. 2001; Morava et al. 2004; Sperl et al. 2006). A subgroup presented with a severe early-onset phenotype with hypertrophic CM, and the unique features of early cataract, hypotonia/developmental delay, and lactic acidosis (Di Rosa et al. 2006). Recently, the underlying genetic defect in a subgroup of patients of Gypsy origin presenting with hypertrophic CM, hypotonia, hepatomegaly, facial dysmorphism, and microcephaly was found. Mutations in TMEM70 encoding a mitochondrial protein proposed to be an ancillary factor involved in the biosynthesis and assembly of adenosine triphosphate (ATP) synthase (complex V of the respiratory chain) cause an isolated deficiency of ATP synthase. Half of the patients died, mostly within the first weeks of life; survivors showed psychomotor and various degrees of mental retardation (Holme et al. 1992; Sperl et al. 2006; Cízková et al. 2008; Honzik et al. 2010). Interestingly, ATP synthase deficiency has been reported twice more in association with 3-MGA-uria. Recently, a young woman with mild mental retardation and peripheral neuropathy was described. She harbored a mutation in the ATP5E gene encoding the F1 epsilon subunit of the ATP synthase. This subunit is supposed to be involved in the incorporation of subunit c to the rotor structure of mammalian ATP synthase (Mayr et al. 2010). Some time ago, a girl with a syndromic phenotype mimicking cerebrooculo-facio-skeletal syndrome (but without microphthalmia/cataracts) was reported. She had hypoplastic kidneys, dysgenesis of the corpus callosum, and progressive brain atrophy involving the basal ganglia. She died aged 14 months after a course with intercurrent infections and seizures. She was found to have a mutation in the ATP12 gene, which encodes an ATP synthase assembly factor (de Meirleir et al. 2004). How these nuclear-encoded mitochondrial disorders involved in ATP synthesis or ATP synthase assembly lead to 3-MGA-uria remains elusive.
In 2006, we reported four patients with a distinct clinical phenotype called MEGDEL association (Wortmann et al. 2006). These patients presented with neuroradiological evidence of Leigh disease, sensorineural hearing loss, recurrent lactic academia, severe neonatal infections, and hypoglycemia. The 3-MGA in urine was moderately elevated, and all patients had complex I deficiency. In the mean time, we found three additional patients from other countries. Genetic investigations are pending. Furthermore, patients with mitochondrial DNA (mtDNA) depletion or deletion syndromes, and m.3243A>G mutation, have been described in literature (POLG1 mutations: de Vries et al. 2007; unspecified mtDNA depletion: Figarella-Branger et al. 1992; Scaglia et al. 2001; Pearson syndrome: Jakobs et al. 1991; Gibson et al. 1992; Lichter-Konecki et al. 1993; m.3243A>G: De Kremer et al. 2001). Recently, we presented a diagnostic strategy that enabled us to elucidate the underlying genetic defect in 11 out of 18 children with 3-MGA type IV by delineating patient groups (encephalomyopathic: SUCLA2; hepatocerebral: POLG1, cardiomyopathic: TMEM70, myopathic: RYR1) on clinical and biochemical grounds (for details, see Wortmann et al. 2009).
The 3-MGA-uria type IV is definitely the most intriguing type of the 3-MGA-urias, with a rapidly broadening spectrum. In contrast to the well-defined, distinct phenotypes 3-MGA-uria I, II, III and V, 3-MGA-uria type IV is most frequently associated with progressive neurological impairment, variable organ dysfunction, and biochemical features of a dysfunctional OXPHOS. Urinary 3-MGA seems to be a biochemical marker for mitochondrial dysfunction.
3-MGA-uria type V (MIM 610198)
The 3-MGA-uria type V, or dilated cardiomyopathy with ataxia (DCMA) syndrome, is a novel autosomal recessive condition with early-onset dilated CM with conduction defects and nonprogressive cerebellar ataxia in 18 patients of the Canadian Dariusleut Hutterite population, further characterized by testicular dysgenesis and growth failure (Davey et al. 2006). Affected patients consistently showed five- to tenfold increases in both plasma and urine 3-MGA and 3-MG. Homozygosity mapping revealed the underlying splice-site mutation in the DNAJC19 gene. Proteins containing a DNAJ domain are typically involved in molecular chaperone systems. Based upon the predicted tertiary structure of DNAJ19 it could be located in the inner mitochondrial membrane. Because of the similarity with the yeast Tim14 protein, a defect of protein import via the inner mitochondrial membrane, as seen in Mohr-Tranebjaerg syndrome, is suggested (Roesch et al. 2002).
Other causes of 3-MGA-uria
Elevated urinary excretion of 3-MGA, parallel with increased excretion of 3-HIVA, 3-MG, 3-methylglutarylcarnitin, and 3-hydroxy-3-methylglutaconic acid, can be found in patients with HMG-CoA lyase deficiency (MIM 246450, Faull et al. 1976). This mitochondrial enzyme catalyses the last step of leucine breakdown (Fig. 1) but is also required for ketogenesis. Patients present with a Reye-like picture with hypoketotic acidosis, metabolic acidosis, and liver failure. The prognosis is good if no damage from the initial presentation remains. The excretion pattern of HMG-CoA lyase deficiency is characteristic and distinguishes it from the other 3-MGA-uria types. Two patients with the late-onset form of multiple acyl-CoA dehydrogenase deficiency (MADD, or glutaric aciduria II, MIM 231680) also excreted 3-MGA (Liang et al. 2009). Deficient electron transfer from the flavin adenine dinucleotide (FAD)-dependent dehydrogenases to the respiratory chain due to genetic defects of electron transfer flavoproteins (ETF) not only affects fatty acid oxidation but also dehydrogenases involved in the metabolism of amino acids (e.g., leucine), which could explain the 3-MGA-uria. The diagnosis is difficult to establish but worth elucidating, as treatment with riboflavin or coenzyme Q10 shows dramatic improvement in some patients.
The 3-MGA-uria can occur secondarily in patients with SLO because of abnormal isoprenoid/cholesterol biosynthesis, as well as in patients with glycogen storage disease Ib (MIM 232220) and IX (MIM306000), where it is speculated that an imbalance between gluconeogenesis and de novo cholesterol synthesis result in secondarily increased 3-MGA excretion (Kelley and Kratz 1995; Law et al. 2003; Wortmann et al. unpublished data). These patients may present with elevated lactate levels and hypoglycemia. However, the clinical picture is distinct enough to allow correct diagnosis.
In the 1990s a letter to the Lancet suggested that 3-MGA-uria seen in pregnant mothers could be indicative for a metabolic disorder or in general a congenital defect in the offspring (De Koning et al. 1996). Investigations in a larger cohort of pregnant women revealed that 3-MGA-uria is frequently seen in healthy pregnant women (13/18 patients reported by Walsh et al. 1997), and no correlation to an underlying disease in the mother or child could be established (Walsh et al. 1997). It is speculated that the 3-MGA-uria occurs due to increased flux via the mevalonate shunt, as this is possible in tissue of ectodermal origin such as the placenta (Walsh et al. 1999). Interestingly, patients with congenital adrenal hyperplasia and thereby elevated production of sterol precursors do not show 3-MGA-uria. The authors concluded that this is due to increased steroid flux occurring in the adrenal cortex, which is of mesodermal origin and where the mevalonate shunt is not supposed to function (Walsh et al. 1999).
Conclusion and approach to the patient with unexplained 3-MGA-uria
In this review, we present to the reader the fascinating spectrum of the 3-MGA-uria syndromes. 3-MGA-uria is an important biochemical marker that should stimulate the physician to proceed with investigations. Therefore, we would end our review by providing an approach to the patient with unexplained 3-MGA-uria. 3-MGA-uria can be seen in two conditions: as a consistent feature in the well-defined 3-MGA-uria subtypes I, II, III, V, and as a marker for mitochondrial dysfunction. It is often necessary to repeat (but certainly worth doing so) the urine organic acid analysis, as the 3-MGA-uria can occur intermittently. Table 1 gives an overview of which diagnostic steps should be taken in patients with 3-MGA-uria and unexplained signs and symptoms, such as optic atrophy or CM. Table 2 provides an overview of all known genes and their translational products that are involved in the 3-MGA-urias.
Abbreviations
- 3-HIVA:
-
3-hydroxyisovaleric acid
- 3-MG:
-
3-methylglutaric acid
- 3-MGA:
-
3-methylglutaconic acid
- 3-MGA-uria:
-
3-methylglutaconic aciduria
- 3-MGH:
-
3-methylglutaconyl-CoA hydratase
- ATP:
-
adenosine triphosphate
- CoA:
-
coenzyme A
- CM:
-
cardiomyopathy
- CSF:
-
cerebrospinal fluid
- DCMA syndrome:
-
dilated cardiomyopathy ataxia syndrome
- ETF:
-
electron transfer flavoproteins
- FAD:
-
flavin adenine dinucleotide
- GC–MS:
-
gas chromatography/mass spectrometry
- HMG-CoA:
-
3-hydroxy-3-methylglutaryl-coenzyme A
- NADP:
-
nicotinamide adenine dinucleotide phosphate
- NADPH:
-
NADP, reduced
- NMR:
-
nuclear magnetic resonance
- OXPHOS:
-
oxidative phosphorylation system
- SD:
-
standard deviation
- SLO:
-
Smith-Lemli-Opitz syndrome
References
Adwani SS, Whitehead BF, Rees PG et al (1997) Heart transplantation for Barth syndrome. Pediatr Cardiol 18(2):143–145
Anikster Y, Kleta R, Shaag A et al (2001) Type III 3-methylglutaconic aciduria (optic atrophy plus syndrome, or Costeff optic atrophy syndrome): identification of the OPA3 gene and its founder mutation in Iraqi Jews. Am J Hum Genet 69(6):1218–1224
Barth PG, Van't Veer-Korthof ET, Van Delden L et al (1981) An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leukocytes. In: Busch HFM, Jennekens FGI, Schotte HR (eds) Mitochondria and Muscular Diseases. Beetsterzwaag: 161–164
Barth PG, Scholte JA, Berden JA, Van der Klei-Van Moorsel JM et al (1983) An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J Neurol Sci 62:327–355
Barth PG, Valianpour F, Bowen VM et al (2004) X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): an update. Am J Med Genet A 126A(4):349–354
Besley GT, Lendon M, Broadhead DM et al (1995) Mitochondrial complex deficiencies in a male with cardiomyopathy and 3-methylglutaconic aciduria. J Inherit Metab Dis 18(2):221–223
Bione S, D'Adamo P, Maestrini E et al (1996) A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat Genet 12(4):385–389
Brennan LE, Nakagawa J, Egger D et al (1999) Characterisation and mitochondrial localisation of AUH, an AU-specific RNA-binding enoyl-CoA hydratase. Gene 228:85–91
Cantlay A, Shokrollahi K, Allen J et al (1999) Genetic analysis of the G4.5 gene in families with suspected Barth syndrome. J Pediatr 135:311–315
Chitayat D, Chemke J, Gibson KM et al (1992) 3-Methylglutaconic aciduria: a marker for as yet unspecified disorders and the relevance of prenatal diagnosis in a ‘new’ type (‘type 4’). J Inherit Metab Dis 15:204–212
Christodoulou J, McInnes R, Jay V et al (1994) Barth syndrome: clinical observations and genetic lnkage studies. Am J Med Genet 50(3):255–264
Cízková A, Stránecký V, Mayr JA et al (2008) TMEM70 mutations cause isolated ATP synthase deficiency and neonatal mitochondrial encephalocardiomyopathy. Nat Genet 40(11):1288–1290
Costeff H, Gadoth N, Apter N et al (1989) A familial syndrome of infantile optic atrophy, movement disorder, and spastic paraplegia. Neurology 39(4):595–597
Dale DC, Bolyard AA, Schwinzer BG et al (2006) The severe chronic neutropenia international registry: 10-year follow-up report. Support Cancer Ther 3(4):220–231
Davey KM, Parboosingh JS, McLeod DR et al (2006) Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, causes DCMA syndrome, a novel autosomal recessive Barth syndrome-like condition. J Med Genet 43(5):385–393
De Koning T, Duran M, Dorland L et al (1996) Maternal 3-methylglutaconic aciduria associated with abnormalities in offspring. Lancet 348(9031):887–888
De Kremer RD, Paschini-Capra A, Bacman S et al (2001) Barth’s syndrome-like disorder: a new phenotype with a maternally inherited A3243G substitution of mitochondrial DNA (MELAS mutation). Am J Med Genet 99(2):83–93
De Meirleir L, Seneca S, Lissens W et al (2004) Respiratory chain complex V deficiency due to a mutation in the assembly gene ATP12. J Med Genet 41(2):120–4
De Vries MC, Rodenburg RJ, Morava E et al (2007) Multiple oxidative phosphorylation deficiencies in severe childhood multi-system disorders due to polymerase gamma (POLG1) mutations. Eur J Pediatr 166(3):229–234
Di Rosa G, Deodato F, Loupatty FJ et al (2006) Hypertrophic cardiomyopathy, cataract, developmental delay, lactic acidosis: a novel subtype of 3-methylglutaconic aciduria. J Inherit Metab Dis 29(4):546–550
Duran M, Beemer FA, Tibosch AS et al (1982). Inherited 3-methylglutaconic aciduria in two brothers--another defect of leucine metabolism. J Pediatr 101(4):551–4
Duran M, Baumgartner ER, Suormala TM et al (1993) Cerebrospinal fluid organic acids in Biotinidase deficiency. J Inherit Metab Dis 16:513–516
Edmond J, Popjak G (1974) Transfer of carbon atoms from mevalonate to n-fatty acids. J Biol Chem 249(1):66–71
Elpeleg ON, Costeff H, Joseph A et al (1994) 3-Methylglutaconic aciduria in the Iraqi-Jewish ‘optic atrophy plus’ (Costeff) syndrome. Dev Med Child Neurol 36(2):167–172
Engelke U, Kremer B, Kluijtmans L et al (2006) NMR spectroscopic studies on the late onset form of 3-methylglutaconic aciduria type I and other defects in leucine metabolism. NMR Biomed 19(2):271–278
Ensenauer R, Muller CB, Schwab KO et al (2000) 3-Methylglutaconyl-CoA hydratase deficiency: a new patient with speech retardation as the leading sign. J Inherit Metab Dis 23:341–344
Eriguchi M, Mizuta H, Kurohara K et al (2006) 3-methylglutaconic aciduria type I causes leukoencephalopathy of adult onset. Neurology 67:1895–1896
Faull K, Bolton P, Halpern B et al (1976) Patient with defect in leucine metabolism. New Eng J Med 294:1013
Figarella-Branger D, Pellissier JF, Scheiner C et al (1992) Defects of the mitochondrial respiratory chain complexes in three pediatric cases with hypotonia and cardiac involvement. J Neurol Sci 108(1):105–113
Gibson KM, Bennett MJ, Mize CE et al (1992) 3-methylglutaconic aciduria associated with Pearson syndrome and respiratory chain defects. J Pediatr 121:940–942
Gibson KM, Wappner RS, Jooste S et al (1998) Variable clinical presentation in three patients with 3-methylglutaconyl-coenzyme A hydratase deficiency. J Inherit Metab Dis 21(6):631–638
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343:425–430
Gunay-Aygun M (2005) 3-methylglutaconic aciduria: a common biochemical marker in various syndromes with diverse clinical features. Mol Genet Metab 84:1–3
Hastings R, Steward C, Tsai-Goodman B et al (2009) Dysmorphology of Barth syndrome. Clin Dysmorphol 18(4):185–187
Ho G, Walter J, Christodoulou J (2008) Costeff optic atrophy syndrome: New clinical case and novel molecular findings. J Inherit Metab Dis. Short Report #134
Holme E, Greter J, Jacobsen CE et al (1992) Mitochondrial ATP-synthase deficiency in a child with 3-methylglutaconic aciduria. Pediatr Res 32(6):731–735
Honzík T, Tesarová M, Mayr JA et al (2010) Mitochondrial encephalocardio-myopathy with early neonatal onset due to TMEM70 mutation. Arch Dis Child 95(4):296–301
Houtkooper RH, Vaz FM (2008) Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci 65(16):2493–2506
Houtkooper RH, Turkenburg M, Poll-The BT et al (2009) The enigmatic role of tafazzin in cardiolipin metabolism. Biochim Biophys Acta 1788(10):2003–2014
Hughes-Fulford M, Feingold KR, Searle GL et al (1986) Role of the kidneys in the metabolism of circulating mevalonate in humans. J Clin Endocrinol Metab 62(6):1227–1231
Huizing M, Dorward H, Ly L et al (2010) OPA3, mutated in 3-methylglutaconic aciduria type III, encodes two transcripts targeted primarily to mitochondria. Mol Genet Metab 100(2):149–154
Ibel H, Endres W, Hadorn HB et al (1993) Multiple respiratory chain abnormalities associated with hypertrophic cardiomyopathy and 3-methylglutaconic aciduria. Eur J Pediatr 152(8):665–670
IJlst L, Loupatty FJ, Ruiter JP et al (2002) 3-Methylglutaconic aciduria type I is caused by mutations in AUH. Am J Hum Genet 71(6):1463–1466
Illsinger S, Lucke T, Zschocke J et al (2004) 3-Methylglutaconic aciduria type I in a boy with fever-associated seizures. Pediatr Neurol 30:213–215
Jakobs C, Danse P, Veerman A (1991) Organic aciduria in Pearson syndrome. Eur J Pediatr 150(9):684
Kelley R, Kratz L (1995) 3-methylglutaconic Acidemia in Smith-Lemli-Opitz syndrome. Pediatr Res 37(5):671–674
Kelley R, Cheatham J, Clark B et al (1991) X-linked dilated cardiomyopathy with neutropenia, growth retardation, and 3-methylglutaconic aciduria. J Pediatr 119:738–747
Kleta R, Skovby F, Christensen E et al (2002) 3-Methylglutaconic aciduria type III in a non-Iraqi-Jewish kindred: clinical and molecular findings. Mol Genet Metab 76(3):201–206
Law LK, Tang NL, Hui J et al (2003) 3-methyglutaconic aciduria in a Chinese patient with glycogen storage disease Ib. J Inherit Metab Dis 26(7):705–709
Leipnitz G, Seminotti B, Amaral AU et al (2008) Induction of oxidative stress by the metabolites accumulating in 3-methylglutaconic aciduria in cerebral cortex of young rats. Life Sci 82:652–662
Liang WC, Ohkuma A, Hayashi YK et al (2009) ETFDH mutations, CoQ10 levels, and respiratory chain activities in patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Neuromuscul Disord 19(3):212–216
Lichter-Konecki U, Trefz F, Rötig A et al (1993) 3-methylglutaconic aciduria in a patient with Pearson syndrome. Eur J Pediatr 152(4):378–379
Ly TB, Peters V, Gibson KM et al (2003) Mutations in the AUH gene cause 3-methylglutaconic aciduria type I. Hum Mutat 21(4):401–407
Lynen F, Knappe J, Lorch E et al (1961) On the biochemical function of biotin. II. Putification and mode of action of beta-methyl-crotonyl-carboxylase. Biochem Z 335:123–167
Mangat J, Lunnon-Wood T, Rees P et al (2007) Successful cardiac transplantation in Barth syndrome–single-centre experience of four patients. Pediatr Transplant 11(3):327–331
Matsumori M, Shoji Y, Takahashi T et al (2005) A molecular lesion in a Japanese patient with severe phenotype of 3-methylglutaconic aciduria type I. Pediatr Int 47:684–686
Mayr JA, Havlícková V, Zimmermann F et al (2010) Mitochondrial ATP synthase deficiency due to a mutation in the ATP5E gene for the F1 {varepsilon} subunit. Hum Mol Genet 19(17):3430–3439
Mazzocco MM, Henry AE, Kelly RI (2007) Barth syndrome is associated with a cognitive phenotype. J Dev Behav Pediatr 28(1):22–30
Morava E, Sengers R, ter Laak H et al (2004) Congenital hypertrophic cardiomyopathy, cataract, mitochondrial myopathy and defective oxidative phosphorylation in two siblings with Sengers-like syndrome. Eur J Pediatr 163(8):467–471
Narisawa K, Gibson KM, Sweetman L et al (1986) Deficiency of 3-methylglutaconyl-coenzyme A hydratase in two siblings with 3-methylglutaconic aciduria. J Clin Invest 77:1148–1152
Neas K, Bennetts B, Carpenter K et al (2005) OPA3 mutation screening in patients with unexplained 3-methylglutaconic aciduria. J Inherit Metab Dis 28(4):525–532
Pei W, Kratz LE, Bernardini I et al (2010) A model of Costeff syndrome reveals metabolic and protective functions of mitochondrial OPA3. Development 137(15):2587–2596
Reynier P, Amati-Bonneau P, Verny C et al (2004) OPA3 gene mutations responsible for autosomal dominant optic atrophy and cataract. J Med Genet 41(9):e110
Roesch K, Curran SP, Tranebjaerg L et al (2002) Human deafness dystonia syndrome is caused by a defect in assembly of the DDP1/TIMM8a-TIMM13 complex. Hum Mol Genet 11(5):477–486
Ruesch S, Krakenbuhl S, Kleinle S et al (1996) Combined 3-methylglutaconic and 3-hydroxy-3-methylglutaric aciduria with endocardial fibroelastosis and dilatative cardiomyopathy in male and female siblings with partial deficiency of complex II/III in fibroblasts. Enzyme Protein 49(5–6):321–329
Ryu SW, Jeong HJ, Choi M, et al (2010) Optic atrophy 3 as a protein of the mitochondrial outer membrane induces mitochondrial fragmentation. Cell Mol Life Sci 67(16):2839–50
Scaglia F, Sutton VR, Bodamer OA et al (2001) Mitochondrial DNA depletion associated with partial complex II and IV deficiencies and 3-methylglutaconic aciduria. J Child Neurol 16(2):136–138
Schmidt MR, Birkebaek N, Gonzalez I, Sunde L (2004) Barth syndrome without 3-methylglutaconic aciduria. Acta Paediatr 93(3):419–421
Schroepfer GJ Jr (1981) Sterol biosynthesis. Annu Rev Biochem 50:585–621
Sperl W, Jesina P, Zeman J et al (2006) Deficiency of mitochondrial ATP synthase of nuclear genetic origin. Neuromuscul Disord 12:821–829
Valianpour F, Wanders RJA, Overmars H et al (2002) Cardiolipin deficiency in X-linked cardioskeletal myopathy and neutropenia (Barth syndrome, MIM 302060): a study in cultured skin fibroblasts. J Pediat 141:729–733
Verny C, Amati-Bonneau P, Dubas F et al (2005) An OPA3 gene mutation is responsible for the disease associating optic atrophy and cataract with extrapyramidal signs. Rev Neurol (Paris) 161:451–454
Walsh R, Conway H, Roche G et al (1997) 3-Methylglutaconic aciduria in pregnancy. Lancet 349(9054):776
Walsh R, Conway H, Roche G, Mayne PD (1999) What is the origin of 3-methylglutaconic acid? J Inherit Metab Dis 22(3):251–255
Weinstock SB, Kopito RR, Endemann G et al (1984) The shunt pathway of mevalonate metabolism in the isolated perfused rat liver. J Biol Chem 259(14):8939–8944
Wortmann S, Rodenburg RJ, Huizing M et al (2006) Association of 3-methylglutaconic aciduria with sensori-neural deafness, encephalopathy, and Leigh-like syndrome (MEGDEL association) in four patients with a disorder of the oxidative phosphorylation. Mol Genet Metab 88(1):47–52
Wortmann SB, Rodenburg RJT, Jonckheere A et al (2009) Biochemical and genetic analysis of 3-methylglutaconic aciduria type IV: a diagnostic strategy. Brain 132(Pt 1):136–146
Wortmann SB, Kremer BH, Graham A et al (2010) 3-Methylglutaconic aciduria type I redefined; a syndrome with late onset leukoencephalopathy. Neurology [in press]
Yen TY, Hwu WL, Chien YH et al (2008) Acute metabolic decompensation and sudden death in Barth syndrome: report of a family and a literature review. Eur J Pediatr 167(8):941–944
Acknowledgements
We thank our colleagues from the Nijmegen Centre for Mitochondrial Disorders (NCMD) at the Department of Pediatrics and the Institute for Genetic and Metabolic Disease (IGMD), and at the Department of Laboratory Medicine in Nijmegen, especially Jan Smeitink, Richard Rodenburg, and Bert van den Heuvel. We thank Richard Kelley (Department of Pediatrics, Johns Hopkins University Medical Center and Division of Metabolism, Kennedy Krieger Institute, Baltimore, MD, USA) and Marjan Huizing (Cell Biology of Metabolic Disorders Unit, National Human Genome Research Institute, Bethesda, MD, USA) for their valuable contributions.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Jörn Oliver Sass
References to electronic databases: 3-methylglutaconic aciduria type I (MIM 250950); 3-methylglutaconic aciduria type II (Barth syndrome, MIM 302060); 3-methylglutaconic aciduria type type III (Costeff syndrome, MIM 258501); 3-methylglutaconic aciduria type IV (MIM 250951); 3-methylglutaconic aciduria type V (DCMA syndrome, MIM 610198); 3-methylglutaconyl-CoA hydratase (EC 4.2.1.18), HMG-CoA lyase deficiency (MIM 246450), Multiple Acyl-CoA dehydrogenase deficiency (MIM 231680), Smith-Lemli-Opitz syndrome (MIM 27400), Glycogen storage disease Ib (MIM 232220) and IX (MIM 306000).
Competing interest: None declared.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wortmann, S.B., Kluijtmans, L.A., Engelke, U.F.H. et al. The 3-methylglutaconic acidurias: what’s new?. J Inherit Metab Dis 35, 13–22 (2012). https://doi.org/10.1007/s10545-010-9210-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-010-9210-7