Abstract
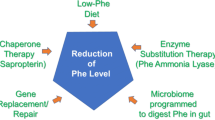
The lecture dedicated to Professor Horst Bickel describes the advances, successes, and opportunities concerning the understanding of the biochemical and molecular basis of phenylketonuria and the innovative treatment strategies introduced for these patients during the last 60 years. These concepts were transferred to other inborn errors of metabolism and led to significant reduction in morbidity and to an improvement in quality of life. Important milestones were the successful development of a low-phenylalanine diet for phenylketonuria patients, the recognition of tetrahydrobiopterin as an option to treat these individuals pharmacologically, and finally market approval of this drug. The work related to the discovery of a pharmacological treatment led metabolic researchers and pediatricians to new insights into the molecular processes linked to mutations in the phenylalanine hydroxylase gene at the cellular and structural level. Again, phenylketonuria became a prototype disorder for a previously underestimated but now rapidly expanding group of diseases: protein misfolding disorders with loss of function. Due to potential general biological mechanisms underlying these disorders, the door may soon open to a systematic development of a new class of pharmaceutical products. These pharmacological chaperones are likely to correct misfolding of proteins involved in numerous genetic and nongenetic diseases.







Similar content being viewed by others
Abbreviations
- PKU:
-
Phenylketonuria
- PAH:
-
Phenylalanine hydroxylase
- BH4 :
-
Tetrahydrobiopterin
- ER:
-
Endoplasmic reticulum
- ERAD:
-
Endoplasmic reticulum associated degradation
References
Aguado C, Pérez B, Ugarte M, Desviat LR (2006) Analysis of the effect of tetrahydrobiopterin on PAH gene expression in hepatoma cells. FEBS Lett 580:1697–1701
Allen SJ, Crown SE, Handel TM (2007) Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol 25:787–820
Andersen O, Flatmark T, Hough E (2001) High resolution crystal structures of the catalytic domain of human phenylalanine hydroxylase in its catalytically active Fe(II) form and binary complex with tetrahydrobiopterin. J Mol Biol 314:279–291
Andersen OA, Stokka AJ, Flatmark T, Hough E (2003) 2.0A resolution crystal structures of the ternary complexes of human phenylalanine hydroxylase catalytic domain with tetrahydrobiopterin and 3-(2-thienyl)-L-alanine or L-norleucine: substrate specificity and molecular motions related to substrate binding. J Mol Biol 333:747–757
Baric I, Fumic K, Glenn B, Cuk M, Schulze A, Finkelstein JD, James SJ, Mejaski-Bosnjak V, Pazanin L, Pogribny IP, Rados M, Sarnavka V, Scukanec-Spoljar M, Allen RH, Stabler S, Uzelac L, Vugrek O, Wagner C, Zeisel S, Mudd SH (2004) S-adenosylhomocysteine hydrolase deficiency in a human: a genetic disorder of methionine metabolism. Proc Natl Acad Sci USA 101:4234–4239
Beluzic R, Cuk M, Pavkov T, Fumic K, Baric I, Mudd SH, Jurak I, Vugrek O (2006) A single mutation at Tyr143 of human S-adenosylhomocysteine hydrolase renders the enzyme thermosensitive and affects the oxidation state of bound cofactor nicotinamide-adenine dinucleotide. Biochem J 400:245–253
Bernegger C, Blau N (2002) High frequency of tetrahydrobiopterin-responsiveness among hyperphenylalaninemias: a study of 1, 919 patients observed from 1988 to 2002. Mol Genet Metab 77:304–313
Bickel H, Gerrard J, Hickmans EM (1953) Influence of phenylalanine intake on phenylketonuric. Lancet 265:812–813
Bickel H, Gerrard J, Hickmans EM (1954) The influence of phenylalanine intake on the chemistry and behaviour of a phenylketonuric child. Acta Paediatr 43:64–77
Bjørgo E, Knappskog PM, Martinez A, Stevens RC, Flatmark T (1998) Partial characterization and three-dimensional-structural localization of eight mutations in exon 7 of the human phenylalanine hydroxylase gene associated with phenylketonuria. Eur J Biochem 257:1–10
Brodsky JL (2007) The protective and destructive roles played by molecular chaperones during ERAD (endoplasmic-reticulum-associated degradation). Biochem J 404:353–363
Bross P, Jespersen C, Jensen TG, Andresen BS, Kristensen MJ, Winter V, Nandy A, Krautle F, Ghisla S, Bolundi L et al (1995) Effects of two mutations detected in medium chain acyl-CoA dehydrogenase (MCAD)-deficient patients on folding, oligomer assembly, and stability of MCAD enzyme. J Biol Chem 270:10284–10290
Bross P, Pedersen P, Winter V, Nyholm M, Johansen BN, Olsen RK, Corydon MJ, Andresen BS, Eiberg H, Kolvraa S, Gregersen N (1999) A polymorphic variant in the human electron transfer flavoprotein alpha-chain (alpha-T171) displays decreased thermal stability and is overrepresented in very-long-chain acyl-CoA dehydrogenase-deficient patients with mild childhood presentation. Mol Genet Metab 67:138–147
Cohen FE, Kelly JW (2003) Therapeutic approaches to protein-misfolding diseases. Nature 426:905–909
Cooper DN, Ball EV, Stenson PD, Phillips AD, Howells K, Mort ME (2010) The Human Gene Mutation Database. http://www.hgmd.cf.ac.uk/ac/index.php
Daily MD, Gray JJ (2007) Local motions in a benchmark of allosteric proteins. Bioinformatics 399:385–399
Daily MD, Upadhyaya TJ, Gray JJ (2008) Contact rearrangements form coupled networks from local motions in allosteric proteins. Proteins 71:455–466
DePristo MA, Weinreich DM, Hartl DL (2005) Missense meanderings in sequence space: a biophysical view of protein evolution. Nat Rev Genet 6:678–687
Erlandsen H, Pey AL, Gamez A, Perez B, Desviat LR, Aguado C, Koch R, Surendran S, Tyring S, Matalon R, Scriver CR, Ugarte M, Martinez A, Stevens RC (2004) Correction of kinetic and stability defects by tetrahydrobiopterin in phenylketonuria patients with certain phenylalanine hydroxylase mutations. Proc Natl Acad Sci USA 101:16903–16908
Fan JQ, Ishii S, Asano N, Suzuki Y (1999) Accelerated transport and maturation of lysosomal alpha-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med 5:112–115
Feillet F, Clarke L, Meli C, Lipson M, Morris AA, Harmatz P, Mould DR, Green B, Dorenbaum A, Giovannini M, Foehr E (2008) Pharmacokinetics of sapropterin in patients with phenylketonuria. Clin Pharmacokinet 47:817–825
Ficko-Blean E, Stubbs KA, Nemirovsky O, Vocadlo DJ, Boraston AB (2008) Structural and mechanistic insight into the basis of mucopolysaccharidosis IIIB. Proc Natl Acad Sci USA 105:6560–6565
Futerman AH, van Meer G (2004) The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol 5:554–565
Gersting SW, Kemter KF, Staudigl M, Messing DD, Danecka MK, Lagler FB, Sommerhoff CP, Roscher AA, Muntau AC (2008) Loss of function in phenylketonuria is caused by impaired molecular motions and conformational instability. Am J Hum Genet 83:5–17
Gersting SW, Lagler FB, Eichinger A, Kemter KF, Danecka MK, Messing DD, Staudigl M, Domdey KA, Zsifkovits C, Fingerhut R, Glossmann H, Roscher AA, Muntau AC (2010) Pahenu1 is a mouse model for tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency and promotes analysis of the pharmacological chaperone mechanism in vivo. Hum Mol Genet 19:2039–2049
Gjetting T, Petersen M, Guldberg P, Güttler F (2001) In vitro expression of 34 naturally occurring mutant variants of phenylalanine hydroxylase: correlation with metabolic phenotypes and susceptibility toward protein aggregation. Mol Genet Metab 72:132–143
Gregersen N, Olsen RKJ (2010) Disease mechanisms and protein structures in fatty acid oxidation defects. J Inherit Metab Dis. doi:10.1007/s10545-010-9046-1
Gregersen N, Bross P, Vang S, Christensen JH (2006) Protein misfolding and human disease. Annu Rev Genomics Hum Genet 7:103–124
Hennermann JB, Bührer C, Blau N, Vetter B, Mönch E (2005) Long-term treatment with tetrahydrobiopterin increases phenylalanine tolerance in children with severe phenotype of phenylketonuria. Mol Genet Metab 86(Suppl 1):S86–S90
Ignatova Z (2005) Monitoring protein stability in vivo. Microb Cell Fact 6:1–6
Kim S-W, Jung J, Oh H-J, Kim J, Lee K-S, Lee D-H, Park C, Kimm K, Koo SK, Jung S-C (2006) Structural and functional analyses of mutations of the human phenylalanine hydroxylase gene. Clin Chim Acta 365:279–287
Koch J (1997) Robert Guthrie—the PKU story: crusade against mental retardation. Hope Publishing House, Pasadena, CA
Kure S, Hou DC, Ohura T, Iwamoto H, Suzuki S, Sugiyama N, Sakamoto O, Fujii K, Matsubara Y, Narisawa K (1999) Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. J Pediatr 135:375–378
Lässker U, Zschocke J, Blau N, Santer R (2002) Tetrahydrobiopterin responsiveness in phenylketonuria. Two new cases and a review of molecular genetic findings. J Inherit Metab Dis 25:65–70
Lee P, Treacy EP, Crombez E, Wasserstein M, Waber L, Wolff J, Wendel U, Dorenbaum A, Bebchuk J, Christ-Schmidt H, Seashore M, Giovannini M, Burton BK, Morris AA (2008) Safety and efficacy of 22 weeks of treatment with sapropterin dihydrochloride in patients with phenylketonuria. Am J Med Genet 146A:2851–2859
Levy HL, Milanowski A, Chakrapani A, Cleary M, Lee P, Trefz FK, Whitley CB, Feillet F, Feigenbaum AS, Bebchuk JD, Christ-Schmidt H, Dorenbaum A (2007) Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study. Lancet 370:504–510
Lieberman RL, Wustman BA, Huertas P, Powe AC Jr, Pine CW, Khanna R, Schlossmacher MG, Ringe D, Petsko GA (2007) Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol 3:101–107
Lindner M, Steinfeld R, Burgard P, Schulze A, Mayatepek E, Zschocke J (2003) Tetrahydrobiopterin sensitivity in German patients with mild phenylalanine hydroxylase deficiency. Hum Mutat 21:400
Luheshi LM, Dobson CM (2009) Bridging the gap: from protein misfolding to protein misfolding diseases. FEBS Lett 583:2581–2586
Maegawa GH, Tropak M, Buttner J, Stockley T Kok F, Clarke JT, Mahuran DJ (2007) Pyrimethamine as a potential pharmacological chaperone for late-onset forms of GM2 gangliosidosis. J Biol Chem 282:9150–9161
Maier EM, Gersting SW, Kemter KF, Jank JM, Reindl M, Messing DD, Truger MS, Sommerhoff CP, Muntau AC (2009) Protein misfolding is the molecular mechanism underlying MCADD identified in newborn screening. Hum Mol Genet 18:1612–1623
Matsuda J, Suzuki O, Oshima A, Yamamoto Y, Noguchi A, Takimoto K, Itoh M, Matsuzaki Y, Yasuda Y, Ogawa S, Sakata Y, Nanba E, Higaki K, Ogawa Y, Tominaga L, Ohno K, Iwasaki H, Watanabe H, Brady RO, Suzuki Y (2003) Chemical chaperone therapy for brain pathology in G(M1)-gangliosidosis. Proc Natl Acad Sci USA 100:15912–15917
McDonald JD, Bode VC, Dove WF, Shedlovsky A (1990) The use of N-ethyl-N-nitrosourea to produce mouse models for human phenylketonuria and hyperphenylalaninemia. Prog Clin Biol Res 340C:407–413
Mu TW, Ong DS, Wang YJ, Balch WE, Yates JR 3rd, Segatori L, Kelly JW (2008) Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell 134:769–781
Muntau AC, Röschinger W, Habich M, Demmelmair H, Hoffmann B, Sommerhoff CP, Roscher AA (2002) Tetrahydrobiopterin as an alternative treatment for mild phenylketonuria. N Engl J Med 347:2122–2132
O'Reilly LP, Andresen BS, Engel PC (2005) Two novel variants of human medium chain acyl-CoA dehydrogenase (MCAD). K364R, a folding mutation, and R256T, a catalytic-site mutation resulting in a well-folded but totally inactive protein. FEBS J 272:4549–4557
Parenti G, Zuppaldi A, Gabriela Pittis M, Rosaria Tuzzi M, Annunziata I, Meroni G, Porto C, Donaudy F, Rossi B, Rossi M, Filocamo M, Donati A, Bembi B, Ballabio A, Andria G (2007) Pharmacological enhancement of mutated alpha-glucosidase activity in fibroblasts from patients with Pompe disease. Mol Ther 15:508–514
Pedersen CB, Bross P, Winter VS, Corydon TJ, Bolund L, Bartlett K, Vockley J, Gregersen N (2003) Misfolding, degradation, and aggregation of variant proteins. The molecular pathogenesis of short chain acyl-CoA dehydrogenase (SCAD) deficiency. J Biol Chem 278:47449–47458
Pérez B, Desviat LR, Gómez-Puertas P, Martínez A, Stevens RC, Ugarte M (2005) Kinetic and stability analysis of PKU mutations identified in BH4-responsive patients. Mol Genet Metab 86(Suppl 1):S11–S16
Pey AL, Desviat LR, Gámez A, Ugarte M, Pérez B (2003) Phenylketonuria: genotype-phenotype correlations based on expression analysis of structural and functional mutations in PAH. Hum Mutat 21:370–378
Pey AL, Perez B, Desviat LR, Martinez MA, Aguado C, Erlandsen H, Gamez A, Stevens RC, Thorolfsson M, Ugarte M, Martinez A (2004) Mechanisms underlying responsiveness to tetrahydrobiopterin in mild phenylketonuria mutations. Hum Mutat 24:388–399
Pey AL, Stricher F, Serrano L, Martinez A (2007) Predicted effects of missense mutations on native-state stability account for phenotypic outcome in phenylketonuria, a paradigm of misfolding diseases. Am J Hum Genet 81:1006–1024
Pey AL, Ying M, Cremades N, Velazquez-Campoy A, Scherer T, Thöny B, Sancho J, Martinez A (2008) Identification of pharmacological chaperones as potential therapeutic agents to treat phenylketonuria. J Clin Invest 118:2858–2867
Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE (2009) Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 78:959–991
Saijo T, Welch WJ, Tanaka K (1994) Intramitochondrial folding and assembly of medium-chain acyl-CoA dehydrogenase (MCAD). Demonstration of impaired transfer of K304E-variant MCAD from its complex with hsp60 to the native tetramer. J Biol Chem 269:4401–4408
Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW (2002) Chemical chaperones increase the cellular activity of N370S beta-glucosidase: a therapeutic strategy for Gaucher disease. Proc Natl Acad Sci USA 99:15428–15433
Scavelli R, Ding Z, Blau N, Haavik J, Martínez A, Thöny B (2005) Stimulation of hepatic phenylalanine hydroxylase activity but not Pah-mRNA expression upon oral loading of tetrahydrobiopterin in normal mice. Mol Genet Metab 86(Suppl 1):S153–S155
Schmitz M, Alfalah M, Aerts JMFG, Naim HY, Zimmer K-P (2005) Impaired trafficking of mutants of lysosomal glucocerebrosidase in Gaucher’s disease. Int J Biochem Cell Biol 37:2310–2320
Schröder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74:739–789
Shedlovsky A, McDonald JD, Symula D, Dove WF (1993) Mouse models of human phenylketonuria. Genetics 134:1205–1210
Shintaku H, Kure S, Ohura T, Okano Y, Ohwada M, Sugiyama N, Sakura N, Yoshida I, Yoshino M, Matsubara Y, Suzuki K, Aoki K, Kitagawa T (2004) Long-term treatment and diagnosis of tetrahydrobiopterin-responsive hyperphenylalaninemia with a mutant phenylalanine hydroxylase gene. Pediatr Res 55:425–430
Spaapen LJ, Bakker JA, Velter C, Loots W, Rubio-Gozalbo ME, Forget PP, Dorland L, De Koning TJ, Poll-The BT, Ploos van Amstel HK, Bekhof J, Blau N, Duran M (2001) Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency in Dutch neonates. J Inherit Metab Dis 24:352–358
Steinfeld R, Kohlschütter A, Zschocke J, Lindner M, Ullrich K, Lukacs Z (2002) Tetrahydrobiopterin monotherapy for phenylketonuria patients with common mild mutations. Eur J Pediatr 161:403–405
Steinfeld R, Kohlschütter A, Ullrich K, Lukacs Z (2004) Efficiency of long-term tetrahydrobiopterin monotherapy in phenylketonuria. J Inherit Metab Dis 27:449–453
Stokka AJ, Carvalho RN, Barroso JF, Flatmark T (2004) Probing the role of crystallographically defined/predicted hinge-bending regions in the substrate-induced global conformational transition and catalytic activation of human phenylalanine hydroxylase by single-site mutagenesis. J Biol Chem 279:26571–26580
Tekpinar M, Zheng W (2010) Predicting order of conformational changes during protein conformational transitions using an interpolated elastic network model. Proteins 78:2469–2481
Thöny B, Ding Z, Martínez A (2004) Tetrahydrobiopterin protects phenylalanine hydroxylase activity in vivo: implications for tetrahydrobiopterin-responsive hyperphenylalaninemia. FEBS Lett 577:507–511
Thórólfsson M, Teigen K, Martínez A (2003) Activation of phenylalanine hydroxylase: effect of substitutions at Arg68 and Cys237. Biochemistry 42:3419–3428
Trefz FK, Aulela-Scholz C, Blau N (2001) Successful treatment of phenylketonuria with tetrahydrobiopterin. Eur J Pediatr 160:315
Trefz FK, Burton BK, Longo N, Casanova MM, Gruskin DJ, Dorenbaum A, Kakkis ED, Crombez EA, Grange DK, Harmatz P, Lipson MH, Milanowski A, Randolph LM, Vockley J, Whitley CB, Wolff JA, Bebchuk J, Christ-Schmidt H, Hennermann JB (2009) Efficacy of sapropterin dihydrochloride in increasing phenylalanine tolerance in children with phenylketonuria: a phase III, randomized, double-blind, placebo-controlled study. J Pediatr 154:700–707
Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM (2004) Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic 5:821–837
Vembar SS, Brodsky JL (2008) One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol 9:944–957
Wang L, Surendran S, Michals-Matalon K, Bhatia G, Tanskley S, Koch R, Grady J, Tyring SK, Stevens RC, Guttler F, Matalon R (2007) Mutations in the regulatory domain of phenylalanine hydroxylase and response to tetrahydrobiopterin. Genet Test 11:174–178
Waters PJ (2003) How PAH gene mutations cause hyper-phenylalaninemia and why mechanism matters: insights from in vitro expression. Hum Mutat 21:357–369
Waters PJ, Parniak MA, Akerman BR, Scriver CR (2000) Characterization of phenylketonuria missense substitutions, distant from the phenylalanine hydroxylase active site, illustrates a paradigm for mechanism and potential modulation of phenotype. Mol Genet Metab 69:101–110
Waters PJ, Scriver CR, Parniak MA (2001) Homomeric and heteromeric interactions between wild-type and mutant phenylalanine hydroxylase subunits: evaluation of two-hybrid approaches for functional analysis of mutations causing hyperphenylalaninemia. Mol Genet Metab 73:230–238
Weglage J, Grenzebach M, van Teeffelen-Heithoff A, Marquardt T, Feldmann R, Denecke J, Gödde D, Koch HG (2002) Tetrahydrobiopterin responsiveness in a large series of phenylketonuria patients. J Inherit Metab Dis 25:321–322
Yu Z, Sawkar AR, Kelly JW (2007) Pharmacologic chaperoning as a strategy to treat Gaucher disease. FEBS J 274:4944–4950
Zhong Q, Simonis N, Li QR, Charloteaux B, Heuze F, Klitgord N, Tam S, Szeto D, Lin C, Hao T, Fan C, Milstein S, Dupuy D, Brasseur R, Hill DE, Cusick ME, Vidal M (2009) Edgetic perturbation models of human inherited disorders. Mol Syst Biol 5:321
Zurflüh MR, Zschocke J, Lindner M, Feillet F, Chery C, Burlina A, Stevens RC, Thöny B, Blau N (2008) Molecular genetics of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Hum Mutat 29:167–175
Acknowledgments
We wish to thank our patients and their families. We are grateful to Anita MacDonald for linguistic revision of the manuscript. We would like to express our gratitude to all our coworkers who contributed to the ongoing research behind this work.
Details of funding
This work was supported by the Bavarian Genome Research Network, BayGene.
Competing interests
Ania C. Muntau is a member of the scientific advisory board of Merck Serono S.A., Geneva, Switzerland, regarding treatment for phenylketonuria.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Jerry Vockley
The Bickel Memorial Lecture 2009, held on the occasion of the International SHS Symposium “Homocysteine, Folate and Cobalamin Disorders”, Fulda, Germany, November 11-13, 2009
Rights and permissions
About this article
Cite this article
Muntau, A.C., Gersting, S.W. Phenylketonuria as a model for protein misfolding diseases and for the development of next generation orphan drugs for patients with inborn errors of metabolism. J Inherit Metab Dis 33, 649–658 (2010). https://doi.org/10.1007/s10545-010-9185-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-010-9185-4