Summary
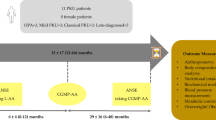
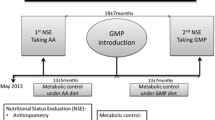
Individuals with phenylketonuria (PKU) must follow a lifelong low-phenylalanine (Phe) diet to prevent neurological impairment. Compliance with the low-Phe diet is often poor owing to restriction in natural foods and the requirement for consumption of a Phe-free amino acid formula or medical food. Glycomacropeptide (GMP), a natural protein produced during cheese-making, is uniquely suited to a low-Phe diet because when isolated from cheese whey it contains minimal Phe (2.5–5 mg Phe/g protein). This paper reviews progress in evaluating the safety, acceptability and efficacy of GMP in the nutritional management of PKU. A variety of foods and beverages can be made with GMP to improve the taste, variety and convenience of the PKU diet. Sensory studies in individuals with PKU demonstrate that GMP foods are acceptable alternatives to amino acid medical foods. Studies in the PKU mouse model demonstrate that GMP supplemented with limiting indispensable amino acids provides a nutritionally adequate source of protein and improves the metabolic phenotype by reducing concentrations of Phe in plasma and brain. A case report in an adult with classical PKU who followed the GMP diet for 10 weeks at home indicates safety, acceptability of GMP food products, a 13–14% reduction in blood Phe levels (p < 0.05) and improved distribution of dietary protein throughout the day compared with the amino acid diet. In summary, food products made with GMP that is supplemented with limiting indispensable amino acids provide a palatable alternative source of protein that may improve dietary compliance and metabolic control of PKU.


Similar content being viewed by others
Abbreviations
- AA:
-
amino acid
- GMP:
-
glycomacropeptide
- LNAA:
-
large neutral amino acids
- MS/MS:
-
tandem mass spectroscopy
- PAH:
-
phenylalanine hydroxylase
- Phe:
-
phenylalanine
- PKU:
-
phenylketonuria
References
Bickel H, Gerrard J, Hickmans EM (1954) The influence of phenylalanine intake on the chemistry and behavior of a phenylketonuric child. Acta Paediatr 43: 64–77.
Brody EP (2000) Biological activities of bovine glycomacropeptide. Br J Nutr 84(Supplement 1): S39–S46.
Bruck WM, Redgrave M, Tuohy KM, et al (2006) Effects of bovine alpha-lactalbumin and casein glycomacropeptide-enriched infant formulae on faecal microbiota in healthy term infants. J Pediatr Gastroenterol Nutr 43: 673–679.
Donlon J, Levy H, Scriver C (2007) Chapter 77: Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Scriver, C, Beaudet A, Sly W, Valle D, eds. Metabolic and Molecular Basis of Inherited Disease. New York: McGraw-Hill. http://www.ommbid.com.
Doultani S, Turhan KN, Etzel MR (2003) Whey protein isolate and glycomacropeptide recovery from whey using ion exchange chromatography. J Food Sci 68: 1389–1395. doi:10.1111/j.1365-2621.2003.tb09655.x.
Etzel MR (2004) Manufacture and use of dairy protein fractions. J Nutr 134: 996S–1002S.
Gregory CO, Yu C, Singh RH (2007) Blood phenylalanine monitoring for dietary compliance among patients with phenylketonuria: comparison of methods. Genet Med 9: 761–765.
Guttler F, Azen C, Guldberg P, et al (1999) Relationship among genotype, biochemical phenotype, and cognitive performance in females with phenylalanine hydroxylase deficiency: report from the Maternal Phenylketonuria Collaborative Study. Pediatrics 104: 258–262. doi:10.1542/peds.104.2.258.
Harper AE, Benevenga NJ, Wohlhueter RM (1970) Effects of ingestion of disproportionate amounts of amino acids. Physiol Rev 50: 428–439.
Hidalgo IJ, Borchardt RT (1990) Transport of a large neutral amino acid (phenylalanine) in a human intestinal epithelial cell line: Caco-2. Biochim Biophys Acta 1028: 25–30. doi:10.1016/0005-2736(90)90261-L.
IOM (2002) Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Protein and Amino Acids (Macronutrients). Washington, DC: National Academy Press.
Keogh JB, Clifton P (2008) The effect of meal replacements high in glycomacropeptide on weight loss and markers of cardiovascular disease risk. Am J Clin Nutr 87: 1602–1605.
Koch R, Moats R, Guttler F, Guldberg P, Nelson M Jr (2000) Blood–brain phenylalanine relationships in persons with phenylketonuria. Pediatrics 106: 1093–1096. doi:10.1542/peds.106.5.1093.
Koch R, Burton B, Hoganson G, et al (2002) Phenylketonuria in adulthood: a collaborative study. J Inherit Metab Dis 25: 333–346. doi:10.1023/A:1020158631102.
Lim K, van Calcar SC, Nelson KL, Gleason ST, Ney DM (2007) Acceptable low-phenylalanine foods and beverages can be made with glycomacropeptide from cheese whey for individuals with PKU. Mol Genet Metab 92: 176–178. doi:10.1016/j.ymgme.2007.06.004.
MacDonald A (2000) Diet and compliance in phenylketonuria. Eur J Pediatr 159(Supplement 2): S136–S141. doi:10.1007/PL00014375.
MacDonald A, Daly A, Davies P, et al (2004) Protein substitutes for PKU: what’s new? J Inherit Metab Dis 27: 363–371. doi:10.1023/B:BOLI.0000031099.79046.65.
MacDonald A, Rylance G, Davies P, Asplin D, Hall SK, Booth IW (2003) Administration of protein substitute and quality of control in phenylketonuria: a randomized study. J Inherit Metab Dis 26: 319–326. doi:10.1023/A:1025186217369.
Matalon R, Surendran S, Matalon KM, et al (2003) Future role of large neutral amino acids in transport of phenylalanine into the brain. Pediatrics 112: 1570–1574.
Matalon R, Michals-Matalon K, Bhatia G, et al (2006) Large neutral amino acids in the treatment of phenylketonuria (PKU). JInherit Metab Dis 29: 732–738. doi:10.1007/s10545-006-0395-8.
Matalon R, Michals-Matalon K, Bhatia G, et al (2007) Double blind placebo control trial of large neutral amino acids in treatment of PKU: effect on blood phenylalanine. J Inherit Metab Dis 30: 153–158. doi:10.1007/s10545-007-0556-4.
McDonald JD, Bode VC, Dove WF, Shedlovsky A (1990) Pahhph-5: a mouse mutant deficient in phenylalanine hydroxylase. Proc Natl Acad Sci U S A 87: 1965–1967. doi:10.1073/pnas.87.5.1965.
Moats RA, Moseley KD, Koch R, Nelson M Jr (2003) Brain phenylalanine concentrations in phenylketonuria: research and treatment of adults. Pediatrics 112: 1575–1579.
Ney DM, Hull AK, van Calcar SC, Liu X, Etzel MR (2008) Dietary glycomacropeptide supports growth and reduces the concentrations of phenylalanine in plasma and brain in a murine model of phenylketonuria. J Nutr 138: 316–322.
NIH (2000) Phenylketonuria (PKU): screening and management. NIH Consensus Statement 17: 1–33.
Pardridge WM (1998) Blood–brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem Res 23: 635–644. doi:10.1023/A:1022482604276.
Pietz J, Kreis R, Rupp A, et al (1999) Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J Clin Invest 103: 1169–1178. doi:10.1172/JCI5017.
Rashed MS, Ozand PT, Bucknall MP, Little D (1995) Diagnosis of inborn errors of metabolism from blood spots by acylcarnitines and amino acids profiling using automated electrospray tandem mass spectrometry. Pediatr Res 38: 324–331.
Sanjurjo P, Aldamiz L, Georgi G, Jelinek J, Ruiz JI, Boehm G (2003) Dietary threonine reduces plasma phenylalanine levels in patients with hyperphenylalaninemia. J Pediatr Gastroenterol Nutr 36: 23–26. doi:10.1097/00005176-200301000-00007.
Schindeler S, Ghosh-Jerath S, Thompson S, et al (2007) The effects of large neutral amino acid supplements in PKU: an MRS and neuropsychological study. Mol Genet Metab 91: 48–54. doi:10.1016/j.ymgme.2007.02.002.
Slocum RH, Cummings JG (1991) Amino acid anaylsis of physiological samples. In Hommes, FA, ed. Techniques in Diagnostic Human Biochemical Genetics: A Laboratory Manual. New York: Wiley-Liss, 87–126
US Department of Agriculture ARS (2005) USDA Nutrient Database for Standard Reference, Release 18. http://www.nal.usda.gov/fnic/foodcomp.
Walter JH, White FJ (2004) Blood phenylalanine control in adolescents with phenylketonuria. Int J Adolesc Med Health 16: 41–45.
Zhao XH, Wen ZM, Meredith CN, Matthews DE, Bier DM, Young VR (1986) Threonine kinetics at graded threonine intakes in young men. Am J Clin Nutr 43: 795–802.
Acknowledgements
This work was supported by the Mid Atlantic Connection for PKU and Allied Disorders, Michaux Family Foundation, Robert Draper Technology Innovation Fund and the USDA Cooperative State Research, Education and Extension Service, project WISO1084, University of Wisconsin-Madison; and by NIH R03-DK-071534 and P30-HD-03352. In addition, the authors acknowledge the assistance of Phillip Williams, Waisman Center Biochemical Genetics Laboratory, for conducting the plasma amino acid analyses; Kimberlee J. Burrington, Coordinator, Dairy Ingredients Applications Group, Wisconsin Center for Dairy Research for her support in developing the GMP food products; and Xiaowen Liu for assistance with statistical analysis. We also acknowledge the GMP Task Force for initially recognizing the potential contribution of GMP to the nutritional management of PKU and facilitating this research. Members of the Task Force include two whey-producing companies, Davisco Foods of Minnesota and Grande Cheese Co. of Wisconsin, and two companies that distribute medical foods for the PKU market, Cambrooke Foods of Boston and Applied Nutrition of New Jersey. Membership also includes faculty and staff of the University of Wisconsin-Madison, parents of children with PKU, and an adult with PKU.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicating editor: Beat Thöny
Competing interests: None declared
References to electronic databases: Phenylalanine hydroxylase: EC 1.14.16.1.
Rights and permissions
About this article
Cite this article
Ney, D.M., Gleason, S.T., van Calcar, S.C. et al. Nutritional management of PKU with glycomacropeptide from cheese whey. J Inherit Metab Dis 32, 32–39 (2009). https://doi.org/10.1007/s10545-008-0952-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-008-0952-4