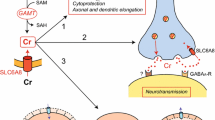
Summary
Creatine deficiency syndromes, either due to AGAT, GAMT or SLC6A8 deficiencies, lead to a complete absence, or a very strong decrease, of creatine within the brain, as measured by magnetic resonance spectroscopy. While the mammalian central nervous system (CNS) expresses AGAT, GAMT and SLC6A8, the lack of SLC6A8 in astrocytes around the blood–brain barrier limits the brain capacity to import creatine from the periphery, and suggests that the CNS has to rely mainly on endogenous creatine synthesis through AGAT and GAMT expression. This seems contradictory with SLC6A8 deficiency, which, despite AGAT and GAMT expression, also leads to creatine deficiency in the CNS. We present novel data showing that in cortical grey matter, AGAT and GAMT are expressed in a dissociated way: e.g. only a few cells co-express both genes. This suggests that to allow synthesis of creatine within the CNS, at least for a significant part of it, guanidinoacetate must be transported from AGAT- to GAMT-expressing cells, possibly through SLC6A8. This would explain the creatine deficiency observed in SLC6A8-deficient patients. By bringing together creatine deficiency syndromes, AGAT, GAMT and SLC6A8 distribution in CNS, as well as a synthetic view on creatine and guanidinoacetate levels in the brain, this review presents a comprehensive framework, including new hypotheses, on brain creatine metabolism and transport, both in normal conditions and in case of creatine deficiency.

Similar content being viewed by others
Abbreviations
- AGAT:
-
L-arginine:glycine amidinotransferase
- BBB:
-
blood–brain barrier
- CAT:
-
cationic amino acid transporter (system y+)
- CK:
-
creatine kinase
- CNS:
-
central nervous system
- Cr:
-
creatine
- CSF:
-
cerebrospinal fluid
- GAA:
-
guanidinoacetate
- GAMT:
-
guanidinoacetate methyltransferase
- MCEC:
-
microcapillary endothelial cell
- MRS:
-
magnetic resonance spectroscopy
- SLC6A8:
-
creatine transporter
- tCr:
-
total creatine (creatine + phosphocreatine)
References
Acosta ML, Kalloniatis M, Christie DL (2005) Creatine transporter localization in developing and adult retina: importance of creatine to retinal function. Am J Physiol Cell Physiol 289: C1015–C1023.
Almeida LS, Verhoeven NM, Roos B, et al (2004) Creatine and guanidinoacetate: diagnostic markers for inborn errors in creatine biosynthesis and transport. Mol Genet Metab 82: 214–219.
Almeida LS, Salomons GS, Hogenboom F, Jakobs C, Schoffelmeer AN (2006) Exocytotic release of creatine in rat brain. Synapse 60: 118–123.
Anselm IM, Alkuraya FS, Salomons GS, et al (2006) X-linked creatine transporter defect: a report on two unrelated boys with a severe clinical phenotype. J Inherit Metab Dis 29: 214–219.
Battini R, Leuzzi V, Carducci C, et al (2002) Creatine depletion in a new case with AGAT deficiency: clinical and genetic study in a large pedigree. Mol Genet Metab 77: 326–331.
Battini R, Alessandri MG, Leuzzi V, et al (2006) Arginine:glycine amidinotransferase (AGAT) deficiency in a newborn: early treatment can prevent phenotypic expression of the disease. J Pediatr 148: 828–830.
Bizzi A, Bugiani M, Salomons GS, et al (2002) X-linked creatine deficiency syndrome: a novel mutation in creatine transporter gene SLC6A8. Ann Neurol 52: 227–231.
Braissant O (2004) Measurement of nitric oxide-related enzymes in the brain by in situ hybridization. Methods Mol Biol 279: 113–124.
Braissant O, Gotoh T, Loup M, Mori M, Bachmann C (1999) L-arginine uptake, the citrulline-NO cycle and arginase II in the rat brain: an in situ hybridization study. Mol Brain Res 70: 231–241.
Braissant O, Gotoh T, Loup M, Mori M, Bachmann C (2001a) Differential expression of the cationic amino acid transporter 2(B) in the adult rat brain. Mol Brain Res 91: 189–195.
Braissant O, Henry H, Loup M, Eilers B, Bachmann C (2001b) Endogenous synthesis and transport of creatine in the rat brain: an in situ hybridization study. Mol Brain Res 86: 193–201.
Braissant O, Henry H, Villard AM, et al (2002) Ammonium-induced impairment of axonal growth is prevented through glial creatine. J Neurosci 22: 9810–9820.
Braissant O, Henry H, Villard AM, Speer O, Wallimann T, Bachmann C (2005) Creatine synthesis and transport during rat embryogenesis: Spatiotemporal expression of AGAT, GAMT and CT1. BMC Dev Biol 5: 9.
Braissant O, Bachmann C, Henry H (2007) Expression and function of AGAT, GAMT and CT1 in the mammalian brain. Subcell Biochem 46: 67–81.
Braissant O, Cagnon L, Monnet-Tschudi F, et al (2008). Ammonium alters creatine transport and synthesis in a 3D-culture of developing brain cells, resulting in secondary cerebral creatine deficiency. Eur J Neurosci doi:10.1111/j.1460-9568.2008.06126.x.
Cagnon L, Braissant O (2007) Hyperammonemia-induced toxicity for the developing central nervous system. Brain Res Rev 56: 183–197.
Caldeira Araujo H, Smit W, et al (2005) Guanidinoacetate methyltransferase deficiency identified in adults and a child with mental retardation. Am J Med Genet A 133: 122–127.
Cecil KM, Salomons GS, Ball WS Jr, et al (2001) Irreversible brain creatine deficiency with elevated serum and urine creatine: acreatine transporter defect? Ann Neurol 49: 401–404.
Cecil KM, DeGrauw TJ, Salomons GS, Jakobs C, Egelhoff JC, Clark JF (2003) Magnetic resonance spectroscopy in a 9-day-old heterozygous female child with creatine transporter deficiency. J Comput Assist Tomogr 27: 44–47.
Daly MM (1985) Guanidinoacetate methyltransferase activity in tissues and cultured cells. Arch Biochem Biophys 236: 576–584.
Dechent P, Pouwels PJ, Wilken B, Hanefeld F, Frahm J (1999) Increase of total creatine in human brain after oral supplementation of creatine-monohydrate. Am J Physiol 277: R698–R704.
DeGrauw TJ, Salomons GS, Cecil KM, et al (2002) Congenital creatine transporter deficiency. Neuropediatrics 33: 232–238.
Dringen R, Verleysdonk S, Hamprecht B, Willker W, Leibfritz D, Brand A (1998) Metabolism of glycine in primary astroglial cells: synthesis of creatine, serine, and glutathione. J Neurochem 70: 835–840.
Ensenauer R, Thiel T, Schwab KO, et al (2004) Guanidinoacetate methyltransferase deficiency: differences of creatine uptake in human brain and muscle. Mol Genet Metab 82: 208–213.
Galbraith RA, Furukawa M, Li M (2006) Possible role of creatine concentrations in the brain in regulating appetite and weight. Brain Res 1101: 85–91.
Ganesan V, Johnson A, Connelly A, Eckhardt S, Surtees RA (1997) Guanidinoacetate methyltransferase deficiency: new clinical features. Pediatr Neurol 17: 155–157.
Guimbal C, Kilimann MW (1993) A Na(+)-dependent creatine transporter in rabbit brain, muscle, heart, and kidney. cDNA cloning and functional expression. J Biol Chem 268: 8418–8421.
Hahn KA, Salomons GS, Tackels-Horne D, et al (2002) X-linked mental retardation with seizures and carrier manifestations is caused by a mutation in the creatine-transporter gene (SLC6A8) located in Xq28. Am J Hum Genet 70: 1349–1356.
Happe HK, Murrin LC (1995) In situ hybridization analysis of CHOT1, a creatine transporter, in the rat central nervous system. J Comp Neurol 351: 94–103.
Hosokawa H, Ninomiya H, Sawamura T, et al (1999) Neuron-specific expression of cationic amino acid transporter 3 in the adult rat brain. Brain Res 838: 158–165.
Item CB, Stöckler-Ipsiroglu S, Stromberger C, et al (2001) Arginine:glycine amidinotransferase deficiency: the third inborn error of creatine metabolism in humans. Am J Hum Genet 69: 1127–1133.
Kan HE, Meeuwissen E, van Asten JJ, Veltien A, Isbrandt D, Heerschap A (2007) Creatine uptake in brain and skeletal muscle of mice lacking guanidinoacetate methyltransferase assessed by magnetic resonance spectroscopy. J Appl Physiol 102: 2121–2127.
Lee H, Kim JH, Chae YJ, Ogawa H, Lee MH, Gerton GL (1998) Creatine synthesis and transport systems in the male rat reproductive tract. Biol Reprod 58: 1437–1444.
Leuzzi V, Bianchi MC, Tosetti M, et al (2000) Brain creatine depletion: guanidinoacetate methyltransferase deficiency (improving with creatine supplementation). Neurology 55: 1407–1409.
Mancardi MM, Caruso U, Schiaffino MC, et al (2007) Severe epilepsy in X-linked creatine transporter defect (CRTR-D). Epilepsia 48: 1211–1213.
Mancini GM, Catsman-Berrevoets CE, de Coo IF, et al (2005) Two novel mutations in SLC6A8 cause creatine transporter defect and distinctive X-linked mental retardation in two unrelated Dutch families. Am J Med Genet A 132: 288–295.
Mercimek-Mahmutoglu S, Stoeckler-Ipsiroglu S, Adami A, et al (2006) GAMT deficiency: features, treatment, and outcome in an inborn error of creatine synthesis. Neurology 67: 480–484.
Möller A, Hamprecht B (1989) Creatine transport in cultured cells of rat and mouse brain. J Neurochem 52: 544–550.
Nakashima T, Tomi M, Katayama K, et al (2004) Blood-to-retina transport of creatine via creatine transporter (CRT) at the rat inner blood-retinal barrier. J Neurochem 89: 1454–1461.
Nakashima T, Tomi M, Tachikawa M, Watanabe M, Terasaki T, Hosoya K (2005) Evidence for creatine biosynthesis in Müller glia. GLIA 52: 47–52.
Neu A, Neuhoff H, Trube G, et al (2002) Activation of GABA(A) receptors by guanidinoacetate: a novel pathophysiological mechanism. Neurobiol Dis 11: 298–307.
Ohtsuki S (2004) New aspects of the blood–brain barrier transporters; its physiological roles in the central nervous system. Biol Pharm Bull 27: 1489–1496.
Ohtsuki S, Tachikawa M, Takanaga H, et al (2002) The blood-brain barrier creatine transporter is a major pathway for supplying creatine to the brain. J Cereb Blood Flow Metab 22: 1327–1335.
Perasso L, Cupello A, Lunardi GL, Principato C, Gandolfo C, Balestrino M (2003) Kinetics of creatine in blood and brain after intraperitoneal injection in the rat. Brain Res 974: 37–42.
Pisano JJ, Abraham D, Udenfriend S (1963) Biosynthesis and disposition of γ-guanidinobutyric acid in mammalian tissues. Arch Biochem Biophys 100: 323–329.
Póo-Argüelles P, Arias A, Vilaseca MA, et al (2006) X-Linked creatine transporter deficiency in two patients with severe mental retardation and autism. J Inherit Metab Dis 29: 220–223.
Renema WK, Schmidt A, van Asten JJ, et al (2003) MR spectroscopy of muscle and brain in guanidinoacetate methyltransferase (GAMT)-deficient mice: validation of an animal model to study creatine deficiency. Magn Reson Med 50: 936–943.
Salomons GS, van Dooren SJ, Verhoeven NM, et al (2001) X-linked creatine-transporter gene (SLC6A8) defect: a newcreatine-deficiency syndrome. Am J Hum Genet 68: 1497–1500.
Salomons GS, van Dooren SJ, Verhoeven NM, et al (2003) X-linked creatine transporter defect: an overview. J Inherit Metab Dis 26: 309–318.
Saltarelli MD, Bauman AL, Moore KR, Bradley CC, Blakely RD (1996) Expression of the rat brain creatine transporter in situ and in transfected HeLa cells. Dev Neurosci 18: 524–534.
Schloss P, Mayser W, Betz H (1994) The putative rat choline transporter CHOT1 transports creatine and is highly expressed in neural and muscle-rich tissues. Biochem Biophys Res Commun 198: 637–645.
Schmidt A, Marescau B, Boehm EA, et al (2004) Severely altered guanidino compound levels, disturbed body weight homeostasis and impaired fertility in a mouse model of guanidinoacetate N-methyltransferase (GAMT) deficiency. Hum Mol Genet 13: 905–921.
Schulze A (2003) Creatine deficiency syndromes. Mol Cell Biochem 244: 143–150.
Schulze A, Battini R (2007) Pre-symptomatic treatment of creatine biosynthesis defects. Subcell Biochem 46: 167–181.
Schulze A, Hess T, Wevers R, et al (1997) Creatine deficiency syndrome caused by guanidinoacetate methyltransferase deficiency: diagnostic tools for a new inborn error of metabolism. J Pediatr 131: 626–631.
Schulze A, Mayatepek E, Bachert P, Marescau B, De Deyn PP, Rating D (1998) Therapeutic trial of arginine restriction in creatine deficiency syndrome. Eur J Pediatr 157: 606–607.
Schulze A, Ebinger F, Rating D, Mayatepek E (2001) Improving treatment of guanidinoacetate methyltransferase deficiency: reduction of guanidinoacetic acid in body fluids by arginine restriction and ornithine supplementation. Mol Genet Metab 74: 413–419.
Schulze A, Bachert P, Schlemmer H, et al (2003) Lack of creatine in muscle and brain in an adult with GAMT deficiency. Ann Neurol 53: 248–251.
Schulze A, Anninos A, Hoffmann GF, et al (2005) AGAT enzyme inhibition by high-dose ornithine: a new approach in treatment of GAMT deficiency. J Inherit Metab Dis 28(Supplement 1): 227.
Schulze A, Hoffmann GF, Bachert P, et al (2006) Presymptomatic treatment of neonatal guanidinoacetate methyltransferase deficiency. Neurology 67: 719–721.
Sijens PE, Verbruggen KT, Oudkerk M, van Spronsen FJ, Soorani-Lunsing RJ (2005) 1H MR spectroscopy of the brain in Cr transporter defect. Mol Genet Metab 86: 421–422.
Stöckler S, Holzbach U, Hanefeld F, et al (1994) Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatr Res 36: 409–413.
Stöckler S, Hanefeld F, Frahm J (1996a) Creatine replacement therapy in guanidinoacetate methyltransferase deficiency, a novel inborn error of metabolism. Lancet 348: 789–790.
Stöckler S, Isbrandt D, Hanefeld F, Schmidt B, Von Figura K (1996b) Guanidinoacetate methyltransferase deficiency: the first inborn error of creatine metabolism in man. Am J Hum Genet 58: 914–922.
Stöckler S, Schutz PW, Salomons GS (2007) Cerebral creatine deficiency syndromes: clinical aspects, treatment and pathophysiology. Subcell Biochem 46: 149–166.
Stromberger C, Bodamer OA, Stöckler-Ipsiroglu S (2003) Clinical characteristics and diagnostic clues in inborn errors of creatine metabolism. J Inherit Metab Dis 26: 299–308.
Struys EA, Jansen EE, Ten Brink HJ, Verhoeven NM, van der Knaap MS, Jakobs C (1998) An accurate stable isotope dilution gas chromatographic-mass spectrometric approach to the diagnosis of guanidinoacetate methyltransferase deficiency. J Pharm Biomed Anal 18: 659–665.
Sykut-Cegielska J, Gradowska W, Mercimek-Mahmutoglu S, Stöckler-Ipsiroglu S (2004) Biochemical and clinical characteristics of creatine deficiencysyndromes. Acta Biochim Pol 51: 875–882.
Tachikawa M, Fukaya M, Terasaki T, Ohtsuki S, Watanabe M (2004) Distinct cellular expressions of creatine synthetic enzyme GAMT and creatine kinases uCK-Mi and CK-B suggest a novel neuron-glial relationship for brain energy homeostasis. Eur J Neurosci 20: 144–160.
Tachikawa M, Hosoya KI, Ohtsuki S, Terasaki T (2007) A novel relationship between creatine transport at the blood-brain and blood-retinal barriers, creatine biosynthesis, and its use for brain and retinal energy homeostasis. Subcell Biochem 46: 83–98.
Torremans A, Marescau B, Possemiers I, et al (2005) Biochemical and behavioural phenotyping of a mouse model for GAMT deficiency. J Neurol Sci 231: 49–55.
Van Pilsum JF, Stephens GC, Taylor D (1972) Distribution of creatine, guanidinoacetate and enzymes for their biosynthesis in the animal kingdom. Implications for phylogeny. Biochem J 126: 325–345.
Virgintino D, Monaghan P, Robertson D, et al (1997) An immunohistochemical and morphometric study on astrocytes and microvasculature in the human cerebral cortex. Histochem J 29: 655–660.
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 281: 21–40.
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80: 1107–1213.
Zugno AI, Scherer EB, Schuck PF, et al (2006) Intrastriatal administration of guanidinoacetate inhibits Na+,K+-ATPase and creatine kinase activities in rat striatum. Metab Brain Dis 21: 41–50.
Acknowledgements
Our work is supported by the Swiss National Science Foundation, grants nos 3100A0-100778 and 3100A0-116859. Theo Wallimann is acknowledged for the anti-AGAT antibody, and Marc Loup for excellent technical work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicating editor: Cornelis Jakobs
Competing interests: None declared
References to electronic databases:L-Arginine:glycine amidinotransferase (AGAT) deficiency: OMIM 602360. Guanidinoacetate N-methyltransferase (GAMT) deficiency: OMIM 601240. Creatine transporter (SLC6A8; Slc6a8) deficiency: OMIM 300352. AGAT: EC 2.1.4.1. GAMT: EC 2.1.1.2.
Rights and permissions
About this article
Cite this article
Braissant, O., Henry, H. AGAT, GAMT and SLC6A8 distribution in the central nervous system, in relation to creatine deficiency syndromes: A review. J Inherit Metab Dis 31, 230–239 (2008). https://doi.org/10.1007/s10545-008-0826-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-008-0826-9