Summary
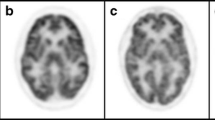
Despite treatment with a galactose-restricted diet, many galactosaemia patients develop lifelong cognitive impairment, speech abnormalities and a gamut of neurological problems including cognitive impairment and tremors. No study has explored changes in cerebral glucose metabolism in patients with galactosaemia. Five patients with galactosaemia had ages ranging from 20 to 40 years (mean age 28 years) and eight similarly aged controls received brain [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) scans. PET scans were analysed using a previously validated template methodology of regions of interest (ROIs). Count ratios for each anatomical ROI were compared between the galactosaemic patients and the healthy controls. Statistical parametric mapping (SPM) software was also used to further analyse the data. ROI analysis showed that galactosaemic patients had significant bilateral decreases in cerebral glucose metabolism in the superior temporal gyrus, medial occipital lobe, parietal lobe, cerebellum, calcarine cortex, superior frontal cortex, and superior parietal cortex when compared with controls. Significant increases were seen in the cingulate gyrus and temporal poles, bilaterally. SPM analysis revealed foci of decreased glucose metabolism in the caudate, cerebellum, precentral gyrus and cerebellar tonsils of galactosaemic patients. SPM also showed increased glucose metabolism in the subcallosal gyrus and claustrum. The results show significant abnormalities in cerebral function in patients with galactosaemia, particularly with widespread decreases in cortical metabolism. These abnormalities appear to be in brain regions that may be associated with the neuropsychological deficits in these patients. PET brain scans may be of value in galactosaemia patients to evaluate for dysfunction.
Similar content being viewed by others
References
al-Essa MA, Rashed MS, Bakheet SM, Patay ZJ, Ozand PT (2000) Glutaric aciduria type II: observations in seven patients with neonatal- and late-onset disease. J Perinatol 20(2): 120–128.
Fridovich-Keil JL (2006) Galactosemia: the good, the bad, and the unknown. J Cell Physiol 209(3): 701–705.
Friston KJ, Ashburner, J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ (1995) Spatial registration and normalization of images. Hum Brain Mapp 3(3): 165–189.
Fukuda M, Mentis MJ, Ma Y, et al (2001) Networks mediating the clinical effects of pallidal brain stimulation for Parkinson’s disease: a PET study of resting-state glucose metabolism. Brain 124(Pt 8): 1601–1609.
Hermann W, Barthel H, Hesse S, et al (2002) Comparison of clinical types of Wilson’s disease and glucose metabolism in extrapyramidal motor brain regions. J Neurol 249(7): 896–901.
Hilker R, Voges J, Weisenbach S, et al (2004) Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson’s disease. J Cereb Blood Flow Metab 24(1): 7–16.
Huang C, Tang C, Feigin A, et al (2007) Changes in network activity with the progression of Parkinson’s disease. Brain 130(Pt 7): 1834–1846.
Karp JS, Surti S, Daube-Witherspoon ME, et al (2003) Performance of a brain PET camera based on anger-logic gadolinium oxyorthosilicate detectors. J Nucl Med 44(8): 1340–1349.
Kaufman FR, Horton EJ, Gott P, et al (1995a) Abnormal somatosensory evoked potentials in patients with classic galactosemia: correlation with neurological outcome. J Child Neurol 10(1): 32–36.
Kaufman FR, McBride-Chang C, Manis FR, Wolff JA, Nelson MD (1995b) Cognitive functioning, neurological status and brain imaging in classical galactosemia. Eur J Pediatr 154(7 Supplement 2): S2–5.
Lancaster JL, Woldorff MG, Parsons LM, et al (2000) Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10(3): 120–131.
Molnár MJ, Valikovics A, Molnár S, et al (2000) Cerebral blood flow and glucose metabolism in mitochondrial disorders. Neurology 55(4): 544–548.
Moore DF, Herscovitch P, Schiffmann R (2001) Selective arterial distribution of cerebral hyperperfusion in Fabry disease. J Neuroimaging 11(3): 303–307.
Nelson CD, Waggoner DD, Donnell GN, Tuerck JM, Buist NR (1991) Verbal dyspraxia in treated galactosemia. Pediatrics 88(2): 346–350.
Nelson MD Jr, Wolff JA, Cross CA, Donnell GN, Kaufman FR. (1992) Galactosemia: evaluation with MR imaging. Radiology 184(1): 255–261.
Newberg AB, Alavi A (2005) The role of PET imaging in the management of patients with central nervous system disorders. Radiol Clin North Am 43(1): 49–65.
Newberg A, Cotter A, Udeshi M, Alavi A, Clark C (2003) A metabolic imaging severity rating scale for the assessment of cognitive impairment. Clin Nucl Med 28(7): 565–570.
Resnick SM, Karp JS, Turetsky B, Gur RE (1993) Comparison of anatomically-defined versus physiologically-based regional localization: effects on PET-FDG quantitation. J Nucl Med 34(12): 2201–2207.
Ridel KR, Leslie ND, Gilbert DL (2005) An updated review of the long-term neurological effects of galactosemia. Pediatr Neurol 33(3): 153–161.
Segal S (1998) Komrower Lecture. Galactosaemia today: the enigma and the challenge. J Inherit Metab Dis 21(5): 455–471.
Signorini M, Paulesu E, Friston K, et al (1999) Rapid assessment of regional cerebral metabolic abnormalities in single subjects with quantitative and nonquantitative [18F]FDG PET: a clinical validation of statistical parametric mapping. Neuroimage 9(1): 63–80.
Talairach J, Tournoux P (1988) Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme.
Waggoner DD, Buist NR, Donnell GN (1990) Long-term prognosis in galactosaemia: results of a survey of 350 cases. J Inherit Metab Dis 13(6): 802–818.
Wang ZJ, Berry GT, Dreha SF, Zhao H, Segal S, Zimmerman RA (2001) Proton magnetic resonance spectroscopy of brain metabolites in galactosemia. Ann Neurol 50(2): 266–269.
Webb AL, Singh RH, Kennedy MJ, Elsas LJ (2003) Verbal dyspraxia and galactosemia. Pediatr Res 53(3): 396–402.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicating editor: Michael Gibson
S. Segal deceased.
Competing interests: None declared
Rights and permissions
About this article
Cite this article
Dubroff, J.G., Ficicioglu, C., Segal, S. et al. FDG-PET findings in patients with galactosaemia. J Inherit Metab Dis 31, 533–539 (2008). https://doi.org/10.1007/s10545-008-0806-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-008-0806-0