Abstract
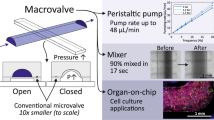
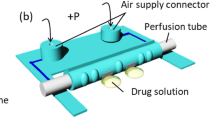
To the extent possible, artificial organs should have characteristics that match those of the in vivo system. To this end, microfabrication techniques allow us to create microenvironments that can help maintain cell organization and functionality in in vitro cultures. We present three new microbioreactors, each of which allows cells to be cultured in a perfused microenvironment similar to that found in vivo. Our microbioreactors use new technology that permits integration onto the chip (35 mm × 20 mm) of an electrical sensor, in addition to one or more pumping systems and associated perfusion circuitry. The monitoring of Caco-2 cell cultures using electrical impedance spectroscopy (EIS) has allowed us to measure the effects of cell growth, cellular barrier formation and the presence of chemical compounds and/or toxins. Specifically, we have investigated the ability of the electrical sensor to maintain appropriate sensitivity and precision. Our results show that the sensor was very sensitive not only to the presence or the absence of the cells, but also to changes in cell state. Our perfused microbioreactors are highly efficient miniaturized tools that are easy to operate. We anticipate that they will offer promising new opportunities in many types of cell culture research, including drug screening and tissue engineering.










Similar content being viewed by others
References
M. Adolphe, G. Barlovatz-Meimon, Culture de Cellules Animales, Methodologies Applications. Editions (INSERM, Paris, France,, 1988)
H. Andersson, A. van den Berg, Lab-on-Chips for Cellomics, Micro and Nano Technologies for Life Sciences (Kluwer, Dordrecht, 2004)
P. Artursson, J. Karlsson, Biochem. Biophys. Res. Commun. 175(3), 880–885 (1991)
F. Asphahani, M. Zhang, Analyst 132, 835–841 (2007)
E. Barsoukov, J.R. Macdonald, Impedance Spectroscopy, 2nd edn. (Wiley, Hoboken, 2005), p. 595
H. Blankson, I. Holen, P.O. Seglen, Exp. Cell Res. 218, 522–530 (1995)
N. Bowden, S. Brittain, A.G. Evans, J.W. Hutchinson, G.M. Whitesides, Nature 393, 146–149 (1998)
Y. Cho, H.S. Kim, A.B. Frazier, Z.G. Chen, D.M. Shin, A. Han, J. Microelectromech. Syst. 18, 808–816 (2009)
A.D. De Lorenzo, A. Andreoli, Curr. Opin. Clin. Nutr. Metab. Care 6, 551–555 (2003)
E. Ekue Creppy, A. Traore, I. Baudrimont, M. Cascante, M.-R. Carratu, Toxicology 181–182, 433–439 (2002)
D. Erickson, D. Li, Anal. Chim. Acta 507, 11–26 (2004)
Y. Feldman, Y. Hayashi, Dielectric Spectroscopy of Biological Systems; from Amino Acids to Cells. XII International conference on Electrical Bio Impedance, 20–24 June. (Gdansk, Poland, 2004), p. 13–16
S. Ferruzza, M.-L. Scarino, G. Rotilio, M.R. Cirilio, P. Santaroni, A. Onetti Muda, Y. Sambuy, Am. J. Physiol.–Gastrointest. 277, 1138–1148 (1999)
S. Ferruzza, M. Scacchi, M.L. Scarino, Y. Sambuy, Toxicol. in Vitro 16, 399–404 (2002)
K.R. Foster, Annu. Rev. Biomed. Eng. 4, 1–27 (2002)
S. Gawad, L. Schild, P. Renaud, Lab Chip 1, 76–82 (2001)
S. Gawad, K. Cheung, U. Seger, A. Bertsch, P. Renaud, Lab Chip 4, 241–251 (2004)
G.O. Gey, Am J Cancer 17, 752–756 (1933)
I. Giaever, C.R. Keese, PNAS 88, 7896–7900 (1991)
I. Giaever, C.R. Keese, Nature 366, 591–592 (1993)
R. Gudivaka, D.A. Schoeller, R.F. Kushner, M.J. Bolt, J. Appl. Physiol. 87, 1087–1096 (1999)
A. Han, L. Yang, A.B. Frazier, Clin. Cancer Res. 13, 139–143 (2007)
D. Holmes, D. Pettigrew, C.H. Reccius, J.D. Gwyer, C.V. Berkel, J. Holloway, D.E. Davie, H. Morgan, Lab Chip 9, 2881–2889 (2009)
C.D. James, N. Reuel, E.S. Lee, R.V. Davalos, S.S. Mani, A. Carroll-Portillo, R. Rebeil, A. Martino, C.A. Apblett, Biosens. Bioelectron. 23, 845–851 (2008)
L.-S. Jang, M.-H. Wang, Biomed. Microdevices 9, 737–743 (2007)
E. Katz, I. Willner, Electroanalysis 15(11), 913–947 (2003)
C.R. Keese, J. Wegener, S.R. Walker, I. Giaever, PNAS 101, 1554–1559 (2004)
S. Kumar, C. Wittman, E. Heinzle, Biotechnol. Lett. 26, 1–10 (2004)
C. Kuttel, E. Nascimento, N. Demierre, T. Silva, T. Braschler, P. Renaud, A.G. Oliva, Acta Trop. 102, 63–68 (2007)
U.G. Kyle, I. Bosaeus, A.D. De Lorenzo, P. Deurenberg, M. Elia, J.M. Gomez, B.L. Heitmann, L. Kent-Smith, J.C. Melchior, M. Pirlich, H. Scharfetter, A.M.W.J. Schols, C. Pichard, Clin. Nutr. 23, 1226–1243 (2004)
E.L. LeCluyse, P.L. Bullock, A. Parkinson, Adv. Drug Deliv. Rev. 22, 133–186 (1996)
F. Leira, C. Alvarez, A.G. Gabado, J.M. Vieites, M.R. Vieytes, L.M. Botana, Anal. Biochem. 317, 129–135 (2003)
P. Linderholm, T. Braschler, J. Vannod, Y. Barrandon, M. Brouard, P. Renaud, Lab Chip 6, 1155–1162 (2006)
D. Malleo, J.T. Nevill, L.P. Lee, H. Morgan, Microfluid Nanofluid 9, 191–198 (2010)
J.C. Mc Donald, D.C. Duffy, J.R. Anderson, D.T. Chiu, H. Wu, O.J.A. Schueller, G.M. Whitesides, Electrophoresis 21, 27–40 (2000)
S. Miret, L. Abrahamse, E.M. Groene, J. Biomol. Screen. 9(7), 598–606 (2004)
H. Morgan, T. Sun, D. Holmes, S. Gawad, N.G. Green, J. Phys. D: Appl. Phys. 40, 61–70 (2007)
A. Moscona, Exp. Cell Res. 3, 535–539 (1952)
Y. Murakami, Y. Oshima, T. Yasumoto, Bull. Jap. Soc. Sci. Fish. 48, 69–72 (1982)
T. Okada, A. Narai, S. Matsunaga, N. Fusetani, M. Shimizu, Toxicol. in Vitro 14, 219–226 (2000)
S. Ostrovidov, J. Jiang, Y. Sakai, T. Fujii, Biomed. Microdevices 6(4), 279–287 (2004)
S. Ostrovidov, J. Mizuno, H. Nakamura, H. Inui, Y. Sakai, T. Fujii, Proceedings of MicroTas 2005, volume 1. (Boston, Massachussetts USA, October 9 – 13, 2005), p. 361
S. Perichon Lacour, S. Wagner, Z. Huang, Z. Suo, Appl. Phys. Lett. 82(15), 2404–2406 (2003)
G. Ranaldi, R. Consalvo, Y. Sambuy, M.L. Scarino, Toxicol. in Vitro 17, 761–767 (2003)
K. Rissanen, S. Ostrovidov, E. Lennon, V. Senez, J. Kim, B. Kim, K. Sakai Furukawa, T. Ushida, Y. Sakai, T. Fujii, Proceedings of 3rd International IEEE EMBS Special Topic Conference on Microtechnologies. (Ohau, Hawai USA, May 12 – 15, 2005), pp. 201–204
R. Rodriguez-Trujillo, O. Castillo-Fernandez, M. Garrido, M. Arundell, A. Valencia, G. Gomila, Biosens. Bioelectron. 24, 290–296 (2008)
G. Schade-Kampmann, A. Huwiler, M. Hebeisen, T. Hessler, M. Di Berardino, Cell Prolif. 41, 830–840 (2008)
H.P. Schwan, Electrical properties of tissue and cell suspensions, in Advances in Biological and Medical Physics, 5, ed. by J.H. Lawrence, C.A. Tobias (Academic, New-York, 1957), pp. 147–209
V. Senez, E. Lennon, S. Ostrovidov, T. Yamamoto, H. Fujita, Y. Sakai, T. Fujii, IEEE Sensor J. 8, 548–557 (2008)
T. Sun, H. Morgan, Microfluid Nanofluid 8, 423–443 (2010)
K. Tachibana, P.J. Scheurer, Y. Tsukitani, H. Kikuchi, D.V. Engen, J. Clardy, Y. Gopichand, F.J. Schimitz, J. Am. Chem. Soc. 103, 2469–2471 (1981)
A. Thomasset, Lyon Med. 209, 1325–1352 (1963)
A. Traore, I. Baudrimont, S. Dano, A. Sanni, Y. Larondelle, Y.J. Schneider, E. Ekue Creppy, Arch. Toxicol. 77(11), 657–662 (2003)
C. Vale, L.M. Botana, FEBS J. 275, 6060–6066 (2008)
C.H. Von Bonsdorff, S.D. Fuller, K. Simons, EMBO J. 4, 2781–2792 (1985)
A.L. Wang, F.L. Wang, H. Yin, W. Xing, Z. Yu, M. Guo, J. Cheng, Biosens. Bioelectron. 25, 990–995 (2010)
B. Xi, N. Yu, X. Wang, X. Xu, Y.A. Abassi, Biotechnol. J. 3, 484–495 (2008)
S. Yamashita, T. Furubayashi, M. Kataoka, T. Sakane, H. Sezaki, H. Tokuda, Eur. J. Pharm. Sci. 10, 195–204 (2000)
J.H. Yeon, J.-K. Park, Anal. Biochem. 341, 308–315 (2005)
Acknowledgments
This project was supported in part by LIMMS (the Laboratory for Integrated Micro Mechatronic Systems) in Tokyo, which is a joint effort of the Communication and Information Science and Technology Department of CNRS (CNRS-STIC, France) and the Institute of Industrial Science (IIS) at the University of Tokyo. It was supported by the CNRS, the MEXT (Japanese Ministry of Education, Culture, Sports, Science and Technology), and the JSPS (Japanese Society for the Promotion of Science). The authors wish to thank Pentax Corporation for their support. We also acknowledge Dr Poleni for his fruitful insights into fluorescence observations with Hoescht and Calcein-AM dyes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ostrovidov, S., Sakai, Y. & Fujii, T. Integration of a pump and an electrical sensor into a membrane-based PDMS microbioreactor for cell culture and drug testing. Biomed Microdevices 13, 847–864 (2011). https://doi.org/10.1007/s10544-011-9555-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-011-9555-1