Abstract
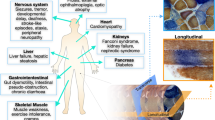
An extensive range of molecular defects have been identified in the human mitochondrial genome (mtDNA), causing a range of clinical phenotypes characterized by mitochondrial respiratory chain dysfunction. Sadly, given the complexities of mitochondrial genetics, there are no available cures for mtDNA disorders. In this review, we consider experimental, genetic-based strategies that have been or are being explored towards developing treatments, focussing on two specific areas which we are actively pursuing—assessing the benefit of exercise training for patients with mtDNA defects, and the prevention of mtDNA disease transmission.


Similar content being viewed by others
References
DiMauro S, Schon EA (2003) Mitochondrial respiratory-chain diseases. N Engl J Med 348:2656–2668
Taylor RW, Turnbull DM (2005) Mitochondrial DNA mutations in human disease. Nat Rev Genet 6:389–402
McFarland R, Taylor RW, Turnbull DM (2002) The neurology of mitochondrial DNA disease. Lancet Neurol 1:345–351
Taylor RW (2005) Gene therapy for the treatment of mitochondrial DNA disorders. Expert Opin Biol Ther 5:183–194
Chinnery P, Majaama K, Turnbull D, Thorburn D (2006) Treatment for mitochondrial disorders. Cochrane Database Syst Rev 1:CD004426
DiMauro, S (2007) Therapeutic strategies—this series of biosciences report
Nagley P, Farrell LB, Gearing DP, Nero D, Meltzer S, Devenish RJ (1988) Assembly of functional proton-translocating ATPase complex in yeast mitochondria with cytoplasmically synthesized subunit 8, a polypeptide normally encoded within the organelle. Proc Natl Acad Sci USA 85:2091–2095
Manfredi G, Fu J, Ojaimi J, Sadlock JE, Kwong JQ, Guy J, Schon EA (2002) Rescue of a deficiency in ATP synthesis by transfer of MTATP6, a mitochondrial DNA-encoded gene, to the nucleus. Nat Genet 30:394–399
Guy J, Qi X, Pallotti F, Schon EA, Manfredi G, Carelli V, Martinuzzi A, Hauswirth WW, Lewin AS (2002) Rescue of a mitochondrial deficiency causing leber hereditary optic neuropathy. Ann Neurol 52:534–542
Funes S, Davidson E, Claros MG, van Lis R, Perez-Martinez X, Vazquez-Acevedo M, King MP, Gonzalez-Halphen D (2002) The typically mitochondrial DNA-encoded ATP6 subunit of the F1F0−ATPase is encoded by a nuclear gene in Chlamydomonas reinhardtii. J Biol Chem 277:6051–6058
Ojaimi J, Pan J, Santra S, Snell WJ, Schon EA (2002) An algal nucleus-encoded subunit of mitochondrial ATP synthase rescues a defect in the analogous human mitochondrial-encoded subunit. Mol Biol Cell 13:3836–3844
Seo BB, Wang J, Flotte TR, Yagi T, Matsuno-Yagi A (2000) Use of the NADH-quinone oxidoreductase (NDI1) gene of Saccharomyces cerevisiae as a possible cure for complex I defects in human cells. J Biol Chem 275:37774–37778
Bai Y, Hajek P, Chomyn A, Bai Y, Chan E, Seo BB, Matsuno-Yagi A, Yagi T, Attardi G (2001) Lack of complex I activity in human cells carrying a mutation in MtDNA-encoded ND4 subunit is corrected by the Saccharomyces cerevisiae NADH-quinone oxidoreductase (NDI1) gene. J Biol Chem 276:38808–38813
Seo BB, Nakamaru-Ogiso E, Cruz P, Flotte TR, Yagi T, Matsuno-Yagi A (2004) Functional expression of the single subunit NADH dehydrogenase in mitochondria in vivo: a potential therapy for complex I deficiencies. Hum Gene Ther 15:887–895
Kolesnikova OA, Entelis NS, Mireau H, Fox TD, Martin RP, Tarassov IA (2000) Suppression of mutations in mitochondrial DNA by tRNAs imported from the cytoplasm. Science 289:1931–1933
Kolesnikova OA, Entelis NS, Jacquin-Becker C, Goltzene F, Chrzanowska-Lightowlers ZM, Lightowlers RN, Martin RP, Tarassov I (2004) DNA-encoded tRNAs targeted into mitochondria can rescue a mitochondrial DNA mutation associated with the MERRF syndrome in cultured human cells. Hum Mol Genet 13:2519–2534
Mahata B, Mukherjee S, Mishra S, Bandyopadhyay A, Adhya S (2006) Functional delivery of a cytosolic tRNA into mutant mitochondria of human cells. Science 314:471–474
Taylor RW, Wardell TM, Connolly BA, Turnbull DM, Lightowlers RN (2001) Linked oligodeoxynucleotides show binding cooperativity and can selectively impair replication of deleted mitochondrial DNA templates. Nucleic Acids Res 29:3404–3412
Taylor RW, Chinnery PF, Turnbull DM, Lightowlers RN (1997) Selective inhibition of mutant human mitochondrial DNA replication in vitro by peptide nucleic acids. Nat Genet 15:212–215
Chinnery PF, Taylor RW, Diekert K, Lill R, Turnbull DM, Lightowlers RN (1999) Peptide nucleic acid delivery to human mitochondria. Gene Ther 6:1919–1928
Muratovska A, Lightowlers RN, Taylor RW, Turnbull DM, Smith RA, Wilce JA, Martin SW, Murphy MP (2001) Targeting peptide nucleic acid (PNA) oligomers to mitochondria within cells by conjugation to lipophilic cations: implications for mitochondrial DNA replication, expression and disease. Nucleic Acids Res 29:1852–1863
Minczuk M, Papworth MA, Kolasinska P, Murphy MP, Klug A (2006) Sequence-specific modification of mitochondrial DNA using a chimeric zinc finger methylase. Proc Natl Acad Sci USA 103:9689–9694
Tanaka M, Borgeld HJ, Zhang J, Muramatsu S, Gong JS, Yoneda M, Maruyama WK, Akao Y, Ohishi N, Miyabayashi S, Umemoto H, Muramatsu T, Furukawa K, Kikuchi A, Nakano I, Ozawa K, Yagi K (2002) Gene therapy for mitochondrial disease by delivering restriction endonuclease SmaI into mitochondria. J Biomed Sci 9:534–541
Srivastava S, Moraes CT (2001) Manipulating mitochondrial DNA heteroplasmy by a mitochondrially targeted restriction endonuclease. Hum Mol Genet 10:3093–3099
Bayona-Bafaluy MP, Blits B, Battersby BJ, Shoubridge EA, Moraes CT (2005) Rapid directional shift of mitochondrial DNA heteroplasmy in animal tissues by a mitochondrially targeted restriction endonuclease. Proc Natl Acad Sci USA 102:14392–14397
Manfredi G, Gupta N, Vazquez-Memije ME, Sadlock JE, Spinazzola A, De Vivo DC, Schon EA (1999) Oligomycin induces a decrease in the cellular content of a pathogenic mutation in the human mitochondrial ATPase 6 gene. J Biol Chem 274:9386–9391
Santra S, Gilkerson RW, Davidson M, Schon EA (2004) Ketogenic treatment reduces deleted mitochondrial DNAs in cultured human cells. Ann Neurol 56:662–669
Taivassalo T, Haller RG (2004) Implications of exercise training in mtDNA defects-use it or lose it? Biochim Biophys Acta 1659:221–231
Taivassalo T, Matthews PM, De Stefano N, Sripathi N, Genge A, Karpati G, Arnold DL (1996) Combined aerobic training and dichloroacetate improve exercise capacity and indices of aerobic metabolism in muscle cytochrome oxidase deficiency. Neurology 47:529–534
Taivassalo T, De Stefano N, Argov Z, Matthews PM, Chen J, Genge A, Karpati G, Arnold DL (1998) Effects of aerobic training in patients with mitochondrial myopathies. Neurology 50:1055–1060
Taivassalo T, De Stefano N, Chen J, Karpati G, Arnold DL, Argov Z (1999) Short-term aerobic training response in chronic myopathies. Muscle Nerve 22:1239–1243
Taivassalo T, Shoubridge EA, Chen J, Kennaway NG, DiMauro S, Arnold DL, Haller RG (2001) Aerobic conditioning in patients with mitochondrial myopathies: physiological, biochemical, and genetic effects. Ann Neurol 50:133–141
Jeppesen TD, Schwartz M, Olsen DB, Wibrand F, Krag T, Duno M, Hauerslev S, Vissing J (2006) Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain 129:3402–3412
Taivassalo T, Gardner JL, Taylor RW, Schaefer AM, Newman J, Barron MJ, Haller RG, Turnbull DM (2006) Endurance training and detraining in mitochondrial myopathies due to single large-scale mtDNA deletions. Brain 129:3391–3401
Fu K, Hartlen R, Johns T, Genge A, Karpati G, Shoubridge EA (1996) A novel heteroplasmic tRNAleu(CUN) mtDNA point mutation in a sporadic patient with mitochondrial encephalomyopathy segregates rapidly in skeletal muscle and suggests an approach to therapy. Hum Mol Genet 5:1835–1840
Weber K, Wilson JN, Taylor L, Brierley E, Johnson MA, Turnbull DM, Bindoff LA (1997) A new mtDNA mutation showing accumulation with time and restriction to skeletal muscle. Am J Hum Genet 60:373–380
Clark KM, Bindoff LA, Lightowlers RN, Andrews RM, Griffiths PG, Johnson MA, Brierley EJ, Turnbull DM (1997 Reversal of a mitochondrial DNA defect in human skeletal muscle. Nat Genet 16:222–224
Shoubridge EA, Johns T, Karpati G (1997) Complete restoration of a wild-type mtDNA genotype in regenerating muscle fibres in a patient with a tRNA point mutation and mitochondrial encephalomyopathy. Hum Mol Genet 6:2239–2242
Taivassalo T, Fu K, Johns T, Arnold D, Karpati G, Shoubridge EA (1999) Gene shifting: a novel therapy for mitochondrial myopathy. Hum Mol Genet 8:1047–1052
Brown DT, Samuels DC, Michael EM, Turnbull DM, Chinnery PF (2000) Random genetic drift determines the level of mutant mtDNA in human primary oocytes. Am J Hum Genet 68:533–536
White SL, Collins VR, Wolfe R, Cleary MA, Shanske S, DiMauro S, Dahl HH, Thorburn DR (1999) Genetic counseling and prenatal diagnosis for the mitochondrial DNA mutations at nucleotide 8993. Am J Hum Genet 65:474–482
Jacobs LJ, de Coo IF, Nijland JG, Galjaard RJ, Los FJ, Schoonderwoerd K, Niermeijer MF, Geraedts JP, Scholte HR, Smeets HJ (2005) Transmission and prenatal diagnosis of the T9176C mitochondrial DNA mutation. Mol Hum Reprod 11:223–228
Bouchet C, Steffann J, Corcos J, Monnot S, Paquis V, Rotig A, Lebon S, Levy P, Royer G, Giurgea I, Gigarel N, Benachi A, Dumez Y, Munnich A, Bonnefont JP (2006) Prenatal diagnosis of myopathy, encephalopathy, lactic acidosis, and stroke-like syndrome: contribution to understanding mitochondrial DNA segregation during human embryofetal development. J Med Genet 43:788–792
Handyside AH, Kontogianni EH, Hardy K, Winston RM (1990) Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature 344:768–770
Steffann J, Frydman N, Gigarel N, Burlet P, Ray PF, Fanchin R, Feyereisen E, Kerbrat V, Tachdjian G, Bonnefont JP, Frydman R, Munnich, A (2006) Analysis of mtDNA variant segregation during early human embryonic development: a tool for successful NARP preimplantation diagnosis. J Med Genet 43:244–247
Briggs DA, Power NJ, Lamb V, Rutherford AJ, Gosden RG (2000) Amplification of DNA sequences in polar bodies from human oocytes for diagnosis of mitochondrial disease. Lancet 355:1520–1521
Dean NL, Battersby BJ, Ao A, Gosden RG, Tan SL, Shoubridge EA, Molnar MJ (2003) Prospect of preimplantation genetic diagnosis for heritable mitochondrial DNA diseases. Mol Hum Reprod 9:631–638
Thorburn DR, Dahl HH (2001) Mitochondrial disorders: genetics, counseling, prenatal diagnosis and reproductive options. Am J Med Genet 106:102–114
Kagawa Y, Hayashi JI (1997) Gene therapy of mitochondrial diseases using human cytoplasts. Gene Ther 4:6–10
Cohen J, Scott R, Schimmel T, Levron J, Willadsen S (1997) Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet 350:186–187
Templeton A (2002) Ooplasmic transfer—proceed with care. New Engl J Med 346:773–775
Hawes SM, Sapienza C, Latham KE (2002) Ooplasmic donation in humans: the potential for epigenic modifications. Hum Reprod 17:850–852
Brenner CA, Barritt JA, Willadsen S, Cohen J (2000) Mitochondrial DNA heteroplasmy after human ooplasmic transplantation. Fertil Steril 74:573–578
Roberts RM (1999) Prevention of human mitochondrial (mtDNA) disease by nucleus transplantation into an enucleated donor oocyte. Am J Med Genet 87:265–266
McGrath J, Solter D (1983) Nuclear transplantation in the mouse embryo by microsurgery and cell fusion. Science 220:1300–1302
Meirelles FV, Smith LC (1997) Mitochondrial genotype segregation in a mouse heteroplasmic lineage produced by embryonic karyoplast transplantation. Genetics 145:445–451
Meirelles FV, Smith LC (1998) Mitochondrial genotype segregation during preimplantation development in mouse heteroplasmic embryos. Genetics 148:877–883
Sato A, Kono T, Nakada K, Ishikawa K, Inoue S, Yonekawa H, Hayashi J (2005) Gene therapy for progeny of mito-mice carrying pathogenic mtDNA by nuclear transplantation. Proc Natl Acad Sci USA 102:16765–16770
Brown DT, Herbert M, Lamb VK, Chinnery PF, Taylor RW, Lightowlers RN, Craven L, Cree L, Gardner JL, Turnbull DM (2006) Transmission of mitochondrial DNA disorders: possibilities for the future. Lancet 368:87–89
Inoue K, Nakada K, Ogura A, Isobe K, Goto Y, Nonaka I, Hayashi JI (2000) Generation of mice with mitochondrial dysfunction by introducing mouse mtDNA carrying a deletion into zygotes. Nat Genet 26:176–181
Acknowledgements
Work in our laboratory is supported by the Muscular Dystrophy Campaign, Wellcome Trust, Department of Health and the Newcastle upon Tyne Hospitals NHS Foundation Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gardner, J.L., Craven, L., Turnbull, D.M. et al. Experimental Strategies Towards Treating Mitochondrial DNA Disorders. Biosci Rep 27, 139–150 (2007). https://doi.org/10.1007/s10540-007-9042-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10540-007-9042-3