Abstract
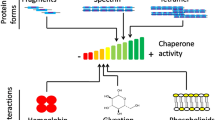
Spectrin is the major constituent protein of the erythrocyte cytoskeleton which forms a filamentous network on the cytoplasmic face of the membrane by providing a scaffold for a variety of proteins. In this review, several aspects of spectrin organization are highlighted, particularly with respect to its ability to bind hydrophobic ligands and its interaction with membrane surfaces. The characteristic binding of the fluorescent hydrophobic probes Prodan and pyrene to spectrin, which allows an estimation of the polarity of the hydrophobic probe binding site, is illustrated. In addition, the contribution of uniquely localized and conserved tryptophan residues in the ‘spectrin repeats’ in these processes is discussed. A functional implication of the presence of hydrophobic binding sites in spectrin is its recently discovered chaperone-like activity. Interestingly, spectrin exhibits residual structural integrity even after denaturation which could be considered as a hallmark of cytoskeletal proteins. Future research could provide useful information about the possible role played by spectrin in cellular physiology in healthy and diseased states.






Similar content being viewed by others
Abbreviations
- 2,3-DPG:
-
2,3-Diphosphoglycerate
- ANS:
-
1-Anilinonapthalene-8-sulfonic acid
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PS:
-
Phosphatidylserine
- PRODAN:
-
6-Propionyl-2-dimethylaminonaphthalene
- REES:
-
Red edge excitation shift
References
An X, Guo X, Sum H, Morrow J, Gratzer W, Mohandas N (2004) Phosphatidylserine binding sites in erythroid spectrin: location and implications for membrane stability. Biochemistry 43:310–315
An X, Guo X, Zhang X, Baines AJ, Debnath G, Moyo D, Salomao M, Bhasin N, Johnson C, Discher D, Gratzer WB, Mohandas N (2006) Conformational stabilities of the structural repeats of erythroid spectrin and their functional implications. J Biol Chem 281:10527–10532
Badcoe IG, Smith CJ, Wood S, Halsall DJ, Holbrook JJ, Lund P, Clarke AR (1991) Binding of a chaperonin to the folding intermediates of lactate dehydrogenase. Biochemistry 30:9195–9200
Balter A, Nowak W, Pawelkiewicz W, Kowalczyk A (1988) Some remarks on the interpretation of the spectral properties of prodan. Chem Phys Lett 143:565–570
Batey S, Randles LG, Steward A, Clarke J (2005) Cooperative folding in a multi-domain protein. J Mol Biol 349:1045–1059
Beaven GH, Gratzer WB (1978) Binding of protoporphyrin and haemin to human spectrin. Acta Haematol 60:321–328
Beck KA, Nelson WJ (1996) The spectrin-based membrane skeleton as a membrane protein-sorting machine. Am J Physiol 270:C1263–C1270
Beechem JM, Brand L (1985) Time-resolved fluorescence of proteins. Annu Rev Biochem 54:43–71
Begg GE, Morris MB, Ralston GB (1997) Comparison of the salt dependent self-association of brain and erythroid spectrin. Biochemistry 36:6977–6985
Bennett V (1990) Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev 70:1029–1065
Bennett V, Gilligan MD (1993) The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol 9:27–66
Bhattacharyya M, Ray S, Bhattacharya S, Chakrabarti A (2004) Chaperone activity and Prodan binding at the self-associating domain of erythroid spectrin. J Biol Chem 279:55080–55088
Bhavesh NS, Panchal SC, Hosur RV (2001) An efficient high-throughput resonance assignment procedure for structural genomics and protein folding research by NMR. Biochemistry 40:14727–14735
Bialkowska K, Zembron A, Sikorski AF (1994) Ankyrin inhibits binding of erythrocyte spectrin to phospholipid vesicles. Biochim Biophys Acta 1191:21–26
Birks JB (1970) Photophysics of aromatic molecules. Wiley-Interscience, London, pp 433–447
Bitbol M, Dempsey C, Watts A, Devaux P (1989) Weak interaction of spectrin with phosphatidylcholine/phosphatidylserine multilayers: a 3H and 31P NMR study. FEBS Lett 244:217–222
Blanco FJ, Serrano L, Forman-Kay JD (1998) High populations of nonnative structures in the denatured state are compatible with the formation of the native folded state. J Mol Biol 284:1153–1164
Buchner J, Schmidt M, Fuchs M, Jaenicke R, Rudolph R, Schmid FX, Kiefhaber T (1991) GroE facilitates refolding of citrate synthase by suppressing aggregation. Biochemistry 30:1586–1591
Cassoly R (1978) Evidence against the binding of native hemoglobin to spectrin of human erythrocytes. FEBS Lett 85:357–360
Chakrabarti A (1996) Fluorescence of spectrin-bound prodan. Biochem Biophys Res Commun 226:495–497
Chakrabarti A, Bhattacharya S (1999) Spectrin exhibits chaperone activity. Curr Sci 77:812–813
Chakrabarti A, Bhattacharya S, Ray S, Bhattacharyya M (2001) Binding of a denatured heme protein and ATP to erythroid spectrin. Biochem Biophys Res Commun 282:1189–1193
Chattopadhyay A (1992) Membrane penetration depth analysis using fluorescence quenching: a critical review. In: Gaber BP, Easwaran KRK (eds) Biomembranes structure & function: the state of the art. Adenine Press, Schenectady, NY, pp 153–163
Chattopadhyay A (2003) Exploring membrane organization and dynamics by the wavelength-selective fluorescence approach. Chem Phys Lipids 122:3–17
Chattopadhyay A, Rawat SS, Kelkar DA, Ray S, Chakrabarti A (2003) Organization and dynamics of tryptophan residues in erythroid spectrin: novel structural features of denatured spectrin revealed by the wavelength-selective fluorescence approach. Protein Sci 12:2389–2403
Cohen AM, Liu S-C, Derick LH, Palek J (1986) Ultrastructural studies of the interaction of spectrin with phosphatidylserine liposomes. Blood 68:920–926
Czogalla A, Kwolek P, Hryniewicz-Jankowska A, Nietubyc M, Leluk J, Sikorski AF (2003) A protein isolated from Escherichia coli, identified as GroEL, reacts with anti-β spectrin antibodies. Arch Biochem Biophys 415:94–100
Datta P, Chakrabarty SB, Chakrabarty A, Chakrabarti A (2003) Interaction of erythroid spectrin with hemoglobin variants: implications in β-thalassemia. Blood Cells Mol Dis 30:248–253
De Matteis MA, Morrow JS (2000) Spectrin tethers and mesh in the biosynthetic pathway. J Cell Sci 113:2331–2343
Delaunay J, Dhermy D (1993) Mutations involving the spectrin heterodimer contact site: clinical expression and alterations in specific function. Semin Hematol 30:21–33
Demchenko AP (1988) Red-edge-excitation fluorescence spectroscopy of singletryptophan proteins. Eur Biophys J 16:121–129
Demchenko AP (2002) The red-edge effects: 30 years of exploration. Luminescence 17:19–42
DeSilva TM, Harper AL, Kotula L, Hensley P, Curtis PJ, Otvos L, Speicher DW (1997) Physical properties of a single-motif erythrocyte spectrin peptide: a highly stable independently folding unit. Biochemistry 36:3991–3997
Diakowski W, Sikorski AF (1995) Interaction of brain spectrin (fodrin) with phospholipids. Biochemistry 34:13252–13258
Diakowski W, Prychidny A, Swistak M, Nietubyc M, Bialkowska K, Szopa J, Sikorski AF (1999) Brain spectrin (fodrin) interacts with phospholipids as revealed by intrinsic fluorescence quenching and monolayer experiments. Biochem J 338:83–90
Diakowski W, Ozimek L, Bielska E, Bem S, Langner M, Sikorski AF (2006) Cholesterol affects spectrin-phospholipid interactions in a manner different from changes resulting from alterations in membrane fluidity due to fatty acyl composition. Biochim Biophys Acta 1758:4–12
Eftink MR (1991) Fluorescence techniques for studying protein structure. In: Suelter CH (ed) Methods of biochemical analysis, vol 35. John Wiley, New York, pp 127–205
Elgsaeter A, Stokke BT, Mikkelsen A, Branton D (1986) The molecular basis of erythrocyte shape. Science 234:1217–1223
Florin-Christensen J, Suarez CE, Florin-Christensen M, Wainszelbaum M, Brown WC, McElwain TF, Palmer GH (2001) A unique phospholipid organization in bovine erythrocyte membranes. Proc Natl Acad Sci USA 98:7733–7741
Gallagher PG, Petruzzi MJ, Weed SA, Zhang Z, Marchesi SL, Mohandas N, Morrow JS, Forget BG (1997) Mutation of a highly conserved residue of βI spectrin associated with fatal and near-fatal neonatal hemolytic anemia. J Clin Invest 99:267–277
Grum VL, Dongning L, MacDonald RI, Mondragón A (1999) Structures of two repeats of spectrin suggest models of flexibility. Cell 98:523–535
Guha S, Rawat SS, Chattopadhyay A, Bhattacharyya B (1996) Tubulin conformation and dynamics: a red edge excitation shift study. Biochemistry 35:13426–13433
Haest CWM (1981) Interactions between membrane skeleton proteins and the intrinsic domain of the erythrocyte membrane. Biochim Biophys Acta 694:331–352
Haque ME, Ray S, Chakrabarti A (2000) Polarity estimate of the hydrophobic binding sites in erythroid spectrin: a study of pyrene fluorescence. J Fluoresc 10:1–6
Hartwig JH (1994) Actin-binding proteins 1: spectrin superfamily. Protein Profile 1:706–778
Hartwig JH (1995) Actin-binding proteins 1: spectrin superfamily. Protein Profile 2:703–800
Isenberg H, Kenna JG, Green NM, Gratzer WB (1981) Binding of hydrophobic ligands to spectrin. FEBS Lett 129:109–112
Johnson RM, Taylor G, Meyer DB (1980) Shape and volume changes in erythrocyte ghosts and spectrin-actin networks. J Cell Biol 86:71–376
Kahana E, Pinder JC, Smith KS, Gratzer WB (1992) Fluorescence quenching of spectrin and other red cell membrane cytoskeletal proteins. Relation to hydrophobic binding sites. Biochem J 282:75–80
Kelkar DA, Chattopadhyay A, Chakrabarti A, Bhattacharyya M (2005) Effect of ionic strength on the organization and dynamics of tryptophan residues in erythroid spectrin: a fluorescence approach. Biopolymers 77:325–334
Klein-Seetharaman J, Oikawa M, Grimshaw SB, Wirmer J, Duchardt E, Ueda T, Imoto T, Smith LJ, Dobson CM, Schwalbe H (2002) Long-range interactions within a nonnative protein. Science 295:1719–1722
Koumanov KS, Tessier C, Momchilova AB, Rainteau D, Wolf C, Quinn PJ (2005) Comparative lipid analysis and structure of detergent-resistant membrane raft fractions isolated from human and ruminant erythrocytes. Arch Biochem Biophys 434:150–158
Kusunoki H, Minasov G, MacDonald RI, Mondragón A (2004) Independent movement, dimerization and stability of tandem repeats of chicken brain α-spectrin. J Mol Biol 344:495–511
LaBrake CC, Wang L, Keiderling TA, Fung LW-M (1993) Fourier transform infrared spectroscopic studies of the secondary structure of spectrin under different ionic strengths. Biochemistry 32:10296–10302
Lange Y, Hadesman RA, Steck TL (1982) Role of the reticulum in the stability and shape of the isolated human erythrocyte membrane. J Cell Biol 92:714–721
Leterrier J-F (2001) Water and the cytoskeleton. Cell Mol Biol 47:901–923
London E, Feigenson GW (1981) Fluorescence quenching in model membranes. 1. Characterization of quenching caused by a spin-labeled phospholipid. Biochemistry 20:1932–1938
Lusitani D, Menhart N, Keiderling TA, Fung LW-M (1998) Ionic strength effect on the thermal unfolding of α-spectrin peptides. Biochemistry 37:16546–16554
MacDonald RI (1993) Temperature and ionic effects on the interaction of erythroid spectrin with phosphatidylserine membranes. Biochemistry 32:6957–6964
MacDonald RI, Musacchio A, Holmgren RA, Saraste M (1994) Invariant tryptophan at a shielded site promotes folding of the conformational unit of spectrin. Proc Natl Acad Sci USA 91:1299–1303
Macgregor RB, Weber G (1986) Estimation of the polarity of the protein interior by optical spectroscopy. Nature 319:70–73
Majee S, Chakrabarti A (1995) A DNA-binding antitumor antibiotic binds to spectrin. Biochem Biophys Res Commun 212:428–432
Majee S, Dasgupta D, Chakrabarti A (1999) Interaction of the DNA-binding antitumor antibiotics, chromomycin and mithramycin with erythroid spectrin. Eur J Biochem 260:619–626
Marchesi VT, Steers E (1968) Selective solubilization of a protein component of the red cell membrane. Science 159:203–204
Markin VS, Kozlov MM (1988) Mechanical properties of the red cell membrane skeleton: analysis of axisymmetric deformations. J Theor Biol 133:147–167
Mazumdar M, Parrack PK, Bhattacharyya B (1992) Interaction of Prodan with tubulin. A fluorescence spectroscopic study. Eur J Biochem 204:127–132
Mc Kiernan AE, MacDonald RI, MacDonald RC, Axelrod D (1997) Cytoskeletal protein binding kinetics at planar phospholipid membranes. Biophys J 73:1987–1998
McGough AM, Josephs R (1990) On the structure of erythrocyte spectrin in partially expanded membrane skeletons. Proc Natl Acad Sci USA 87:5208–5212
Menhart N, Mitchell T, Lusitani D, Topouzian N, Fung LW-M (1996) Peptides with more than one 106-amino acid sequence motif are needed to mimic the structural stability of spectrin. J Biol Chem 271:30410–30416
Mohandas N, Evans E (1994) Mechanochemical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu Rev Biophys Biomol Struct 23:787–818
Mombers C, van Dijck PWM, van Deenen LLM, De Gier J, Verkleij AJ (1977) The interaction of spectrin-actin and synthetic phospholipids. Biochim Biophys Acta 470:152–160
Mombers C, De Gier J, Demel RA, van Deenen LLM (1980) Spectrinphospholipid interaction: a monolayer study. Biochim Biophys Acta 603:52–62
Mondal M, Chakrabarti A (2002) The tertiary amine local anesthetic dibucaine binds to the membrane skeletal protein spectrin. FEBS Lett 532:396–400
Mozo-Villarias A, Morros A, Andreu JM (1991) Thermal transitions in the structure of tubulin. Eur Biophys J 19:295–300
Needleman SB, Wunsch CD (1970) A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol 48:443–453
Neri D, Billeter M, Wider G, Wüthrich K (1992) NMR determination of residual structure in a urea-denatured protein, the 434-repressor. Science 257:1559–1563
Niggli V (2001) Structural properties of lipid-binding sites in cytoskeletal proteins. Trends Biochem Sci 26:604–611
Op den Kamp JAF (1979) Lipid asymmetry in membranes. Annu Rev Biochem 48:47–71
O’Toole PJ, Wolfe C, Ladha S, Cherry RJ (1999) Rapid diffusion of spectrin bound to a lipid surface. Biochim Biophys Acta 1419:64–70
O’Toole PJ, Morrison IEG, Cherry RJ (2000) Investigations of spectrinlipid interactions using fluoresceinphosphatidylethanolamine as a membrane probe. Biochim Biophys Acta 1466:39–46
Pantazatos DP, MacDonald RI (1997) Site-directed mutagenesis of either the highly conserved Trp-22 or the moderately conserved Trp-95 to a large, hydrophobic residue reduces the thermodynamic stability of a spectrin repeating unit. J Biol Chem 272:21052–21059
Pascual J, Pfuhl M, Walther D, Saraste M, Nilges M (1997) Solution structure of the spectrin repeat: a left-handed antiparallel triple-helical coiled-coil. J Mol Biol 273:740–751
Phillips MD, Thomas GH (2006) Brush border spectrin is required for early endosome recycling in Drosophila. J Cell Sci 119:1361–1370
Raghuraman H, Kelkar DA, Chattopadhyay A (2005) Novel insights into protein structure and dynamics utilizing the red edge excitation shift approach. In: Geddes CD, Lakowicz JR (eds) Reviews in fluorescence 2005, vol 2. Plenum Press, New York, NY, pp 199–214
Ralston GB (1991) Temperature and pH dependence of the self-association of human spectrin. Biochemistry 30:4179–4186
Ray S, Chakrabarti A (2003) Erythroid spectrin in micellar detergents. Cell Motil Cytoskeleton 54:16–28
Ray S, Chakrabarti A (2004) Membrane interaction of erythroid spectrin: surface-density-dependent high-affinity binding to phosphatidylethanolamine. Mol Membr Biol 21:93–100
Ropson IJ, Boyer JA, Dalessio PM (2006) A residual structure in unfolded intestinal fatty acid binding protein consists of amino acids that are neighbors in the native state. Biochemistry 45:2608–2617
Sahr KE, Laurila P, Kotula L, Scarpa AL, Coupal E, Leto TL, Linnenbach AJ, Winkelmann JC, Speicher DW, Marchesi VT, Curtis PJ, Forget BG (1990) The complete cDNA and polypeptide sequences of human erythroid α-spectrin. J Biol Chem 265:4434–4443
Samanta A, Fessenden RW (2000) Excited state dipole moment of PRODAN as determined from transient dielectric loss measurements. J Phys Chem A 104:8972–8975
Sayle RA, Milner-White EJ (1995) RasMol: biomolecular graphics for all. Trends Biochem Sci 20:374–376
Shotton DM, Burke BE, Branton D (1979) The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol 131:303–329
Sikorski AF, Michalak K, Bobrowska M (1987) Interaction of spectrin with phospholipids. Quenching of spectrin intrinsic fluorescence by phospholipid suspensions. Biochim Biophys Acta 904:55–60
Sikorski AF, Hanus-Lorenz B, Jezierksi A, Dluzewski AR (2000) Interaction of membrane skeletal protein with membrane lipid domain. Acta Biochim Polonica 47:565–578
Speicher DW, Marchesi VT (1984) Erythrocyte spectrin is compromised of many homologous triple helical segments. Nature 311:177–180
Streichman S, Kahana E, Silver BL (1991) EPR study of the hydrophobic interaction of spectrin with fatty acids. Biochim Biophys Acta 1066:9–13
Subbarao NK, MacDonald RI, Takeshita K, MacDonald RC (1991) Characteristics of spectrin-induced leakage of extruded, phosphatidylserine vescicles. Biochim Biophys Acta 1063:147–154
Subbarao NK, MacDonald RC (1994) Fluorescence studies of spectrin and its subunits. Cell Motil Cytoskeleton 29:72–81
Svetina S, Iglic A, Kraij-Iglic V, Zeks B (1996) Cytoskeleton and red cell shape. Cell Mol Biol Lett 1:67–75
Thomas GH, Newbern EC, Korte CC, Bales MA, Muse SV, Clark AG, Kiehart DP (1997) Intragenic duplication and divergence in the spectrin superfamily of proteins. Mol Biol Evol 14:1285–1295
Turro NJ, Kuo PL, Somasundaran P, Wong K (1986) Surface and bulk interactions of ionic and nonionic surfactants. J Phys Chem 90:288–291
Vertessey BG, Steck TL (1989) Elasticity of the human red cell membrane skeleton. Effects of temperature and denaturants. Biophys J 55:255–262
Viel A (1999) α-Actinin and spectrin structures: an unfolding family story. FEBS Lett 460:391–394
Weber G, Farris FJ (1979) Synthesis and spectral properties of a hydrophobic fluorescent probe: 6-propionyl-2-(dimethylamino)naphthalene. Biochemistry 18:3075–3078
Wichterle H, Hanspal M, Palek J, Jarolim P (1996) Combination of two mutant alpha spectrin alleles underlies a severe spherocytic hemolytic anemia. J Clin Invest 98:2300–2307
Williamson P, Bateman J, Kozarsky K, Mattocks K, Hermanowicz N, Choe H-R, Schlegel RA (1982) Involvement of spectrin in the maintenance of phase-state asymmetry in the erythrocyte membrane. Cell 30:725–733
Winkelmann JC, Forget BG (1993) Eythroid and Nonerythroid Spectrins. Blood 81:3173–3185
Winkelmann JC, Chang J-G, Tse WT, Scarpa AL, Marchesi VT, Forget BG (1990) Full-length sequence of the cDNA for human erythroid β- spectrin. J Biol Chem 265:11827–11832
Acknowledgements
Work in A. Chattopadhyay’s laboratory was supported by a grant (00-141 RG/BIO/AS) from The Third World Academy of Sciences, Trieste, Italy, and by the Council of Scientific and Industrial Research, Government of India. Work in A. Chakrabarti’s laboratory was supported by the Department of Atomic Energy, Government of India. D.A.K. thanks the Life Sciences Research Board for the award of a Postdoctoral Fellowship. A. Chattopadhyay is an Honorary Professor of the Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore (India). Some of the work described in this article was carried out by former members of our research groups. We would like to thank members of the Chattopadhyay laboratory for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chakrabarti, A., Kelkar, D.A. & Chattopadhyay, A. Spectrin Organization and Dynamics: New Insights. Biosci Rep 26, 369–386 (2006). https://doi.org/10.1007/s10540-006-9024-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10540-006-9024-x