Abstract
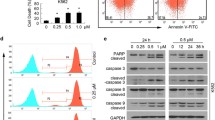
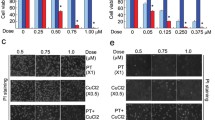
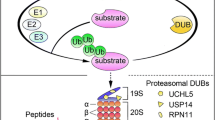
The ubiquitin–proteasome system (UPS) is indispensable to the protein quality control in eukaryotic cells. Due to the remarkable clinical success of using proteasome inhibitors for clinical treatment of multiple myeloma, it is anticipated that targeting the UPS upstream of the proteasome step be an effective strategy for cancer therapy. Deubiquitinases (DUB) are proteases that remove ubiquitin from target proteins and therefore regulate multiple cellular processes including some signaling pathways altered in cancer cells. Thus, targeting DUB is a promising strategy for cancer drug discovery. Previously, we have reported that metal complexes, such as copper and gold complexes, can disrupt the UPS via suppressing the activity of 19S proteasome-associated DUBs and/or of the 20S proteasomes, thereby inducing cancer cell death. In this study, we found that cadmium pyrithione (CdPT) treatment led to remarkable accumulation of ubiquitinated proteins in cultured cancer cells and primary leukemia cells. CdPT potently inhibited the activity of proteasomal DUBs (USP14 and UCHL5), but slightly inhibited 20S proteasome activity. The anti-cancer activity of CdPT was associated with triggering apoptosis via caspase activation. Moreover, treatment with CdPT inhibited proteasome function and repressed tumor growth in animal xenograft models. Our results show that cadmium-containing complex CdPT may function as a novel proteasomal DUB inhibitor and suggest appealing prospects for cancer treatment.







Similar content being viewed by others
References
Adams J (2003) The proteasome: structure, function, and role in the cell. Cancer Treat Rev 29:3–9
Adams J (2004) The development of proteasome inhibitors as anticancer drugs. Cancer Cell 5:417–421
Anderson DJ, Le Moigne R, Djakovic S, Kumar B, Rice J, Wong S, Wang J, Yao B, Valle E, Kiss von Soly S, Madriaga A, Soriano F, Menon MK, Wu ZY, Kampmann M, Chen Y, Weissman JS, Aftab BT, Yakes FM, Shawver L, Zhou HJ, Wustrow D, Rolfe M (2015) Targeting the AAA ATPase p97 as an approach to treat cancer through disruption of protein homeostasis. Cancer Cell 28:653–665
Bruijnincx PC, Sadler PJ (2008) New trends for metal complexes with anticancer activity. Curr Opin Chem Biol 12:197–206
Chen D, Cui QC, Yang H, Dou QP (2006) Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res 66:10425–11433
Chen X, Shi X, Zhao C, Li X, Lan X, Liu S, Huang H, Liu N, Liao S, Zang D, Song W, Liu Q, Carter BZ, Dou QP, Wang X, Liu J (2014) Anti-rheumatic agent auranofin induced apoptosis in chronic myeloid leukemia cells resistant to imatinib through both Bcr/Abl-dependent and -independent mechanisms. Oncotarget 5:9118–9132
Ciechanover A (1994) The ubiquitin-proteasome proteolytic pathway. Cell 9:13–21
Coux O, Tanaka K, Goldberg AL (1996) Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem 65:801–847
Cvek B, Milacic V, Taraba J, Dou QP (2008) Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J Med Chem 51:6256–6258
Daniel KG, Chen D, Orlu S, Cui QC, Miller FR, Dou QP (2005) Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res 7:R897–908
D’Arcy P, Linder S (2012) Proteasome deubiquitinases as novel targets for cancer therapy. Int J Biochem Cell Biol 44:1729–1738
D’Arcy P, Brnjic S, Olofsson MH, Fryknas M, Lindsten K, De Cesare M, Perego P, Sadeghi B, Hassan M, Larsson R, Linder S (2011) Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat Med 17:1636–1640
Dou QP, Li B (1999) Proteasome inhibitors as potential novel anticancer agents. Drug Resist Updates 2:215–223
Fraile JM, Quesada V, Rodriguez D, Freije JM, Lopez-Otin C (2012) Deubiquitinases in cancer: new functions and therapeutic options. Oncogene 31:2373–2388
Frezza M, Hindo S, Chen D, Davenport A, Schmitt S, Tomco D, Dou QP (2010) Novel metals and metal complexes as platforms for cancer therapy. Curr Pharm Des 16:1813–1825
Ho YK, Bargagna-Mohan P, Wehenkel M, Mohan R, Kim KB (2007) LMP2-specific inhibitors: chemical genetic tools for proteasome biology. Chem Biol 14:419–430
Huang H, Zhang X, Li S, Liu N, Lian W, McDowell E, Zhou P, Zhao C, Guo H, Zhang C, Yang C, Wen G, Dong X, Lu L, Ma N, Dong W, Dou QP, Wang X, Liu J (2010) Physiological levels of ATP negatively regulate proteasome function. Cell Res 20:1372–1385
Il’yasova D, Schwartz GG (2005) Cadmium and renal cancer. Toxicol Appl Pharmacol 207:179–186
Jamieson ER, Lippard SJ (1999) Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev 99:2467–2498
Kazi A, Ozcan S, Tecleab A, Sun Y, Lawrence HR, Sebti SM (2014) Discovery of PI-1840, a novel noncovalent and rapidly reversible proteasome inhibitor with anti-tumor activity. J Biol Chem 289:11906–11915
Kisselev AF, Callard A, Goldberg AL (2006) Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem 281:8582–8590
Koulich E, Li X, DeMartino GN (2008) Relative structural and functional roles of multiple deubiquitylating proteins associated with mammalian 26S proteasome. Mol Biol Cell 19:1072–1082
Leon IE, Cadavid-Vargas JF, Di Virgilio AL, Etcheverry S (2016) Vanadium, ruthenium and copper compounds: a new class of non-platinum metallodrugs with anticancer activity. Curr Med Chem 24:112–148
Li L, Yang H, Chen D, Cui C, Dou QP (2008) Disulfiram promotes the conversion of carcinogenic cadmium to a proteasome inhibitor with pro-apoptotic activity in human cancer cells. Toxicol Appl Pharmacol 229:206–214
Liu N, Li X, Huang H, Zhao C, Liao S, Yang C, Liu S, Song W, Lu X, Lan X, Chen X, Yi S, Xu L, Jiang L, Zhao C, Dong X, Zhou P, Li S, Wang S, Shi X, Dou PQ, Wang X, Liu J (2014a) Clinically used antirheumatic agent auranofin is a proteasomal deubiquitinase inhibitor and inhibits tumor growth. Oncotarget 5:5453–5471
Liu N, Liu C, Li X, Liao S, Song W, Yang C, Zhao C, Huang H, Guan L, Zhang P, Liu S, Hua X, Chen X, Zhou P, Lan X, Yi S, Wang S, Wang X, Dou QP, Liu J (2014b) A novel proteasome inhibitor suppresses tumor growth via targeting both 19S proteasome deubiquitinases and 20S proteolytic peptidases. Sci Rep 4:5240
Liu N, Huang H, Dou QP, Liu J (2015) Inhibition of 19S proteasome-associated deubiquitinases by metal-containing compounds. Oncoscience 2:457–466
Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M, Kroemer G (1996) Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med 184:1155–1160
Mollah S, Wertz IE, Phung Q, Arnott D, Dixit VM, Lill JR (2007) Targeted mass spectrometric strategy for global mapping of ubiquitination on proteins. Rapid Commun Mass Spectrom 21:3357–3364
Nair AR, Lee WK, Smeets K, Swennen Q, Sanchez A, Thevenod F, Cuypers A (2015) Glutathione and mitochondria determine acute defense responses and adaptive processes in cadmium-induced oxidative stress and toxicity of the kidney. Arch Toxicol 89:2273–2289
Ott I, Gust R (2007) Non platinum metal complexes as anti-cancer drugs. Arch Pharm 340:117–126
Paramore A, Frantz S (2005) Bortezomib. Nat Rev Drug Discov 2:611–612
Roder C, Thomson MJ (2015) Auranofin: repurposing an old drug for a golden new age. Drugs R D 15:13–20
Shi X, Chen X, Li X, Lan X, Zhao C, Liu S, Huang H, Liu N, Liao S, Song W, Zhou P, Wang S, Xu L, Wang X, Dou QP, Liu J (2014) Gambogic acid induces apoptosis in imatinib-resistant chronic myeloid leukemia cells via inducing proteasome inhibition and caspase-dependent Bcr-Abl downregulation. Clin Cancer Res 20:151–163
Skrott Z, Cvek B (2012) Diethyldithiocarbamate complex with copper: the mechanism of action in cancer cells. Mini Rev Med Chem 12:1184–1192
Tailler M, Senovilla L, Lainey E, Thepot S, Metivier D, Sebert M, Baud V, Billot K, Fenaux P, Galluzzi L, Boehrer S, Kroemer G, Kepp O (2012) Antineoplastic activity of ouabain and pyrithione zinc in acute myeloid leukemia. Oncogene 31:3536–3546
Thompson KH, Orvig C (2006) Metal complexes in medicinal chemistry: new vistas and challenges in drug design. Dalton Trans 6:761–764
Verani CN (2012) Metal complexes as inhibitors of the 26S proteasome in tumor cells. J Inorg Biochem 106:59–67
Verma R, Aravind L, Oania R, McDonald WH, Yates JR III, Koonin EV, Deshaies RJ (2002) Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science 298:611–615
Wei R, Liu X, Yu W, Yang T, Cai W, Liu J, Huang X, Xu GT, Zhao S, Yang J, Liu S (2015) Deubiquitinases in cancer. Oncotarget 6:12872–12889
Wu WK, Cho CH, Lee CW, Wu K, Fan D, Yu J, Sung JJ (2010) Proteasome inhibition: a new therapeutic strategy to cancer treatment. Cancer Lett 293:15–22
Zhang Z, Bi C, Buac D, Fan Y, Zhang X, Zuo J, Zhang P, Zhang N, Dong L, Dou QP (2013) Organic cadmium complexes as proteasome inhibitors and apoptosis inducers in human breast cancer cells. J Inorg Biochem 123:1–10
Zhao C, Chen X, Zang D, Lan X, Liao S, Yang C, Zhang P, Wu J, Li X, Liu N, Liao Y, Huang H, Shi X, Jiang L, Liu X, He Z, Dou QP, Wang X, Liu J (2016a) A novel nickel complex works as a proteasomal deubiquitinase inhibitor for cancer therapy. Oncogene 35:5916–5927
Zhao C, Chen X, Zang D, Lan X, Liao S, Yang C, Zhang P, Wu J, Li X, Liu N, Liao Y, Huang H, Shi X, Jiang L, Liu X, He Z, Wang X, Liu J (2016b) Platinum-containing compound platinum pyrithione is stronger and safer than cisplatin in cancer therapy. Biochem Pharmacol 116:22–38
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (2006AA02Z4B5), NSFC (81472762/H1609), MOE (20134423110002), Central Financial Grant of China (B16056001) (to J.L.), by Foundation for Young Innovative Talents of Guangdong Province (2016KQNCX136) and Guangdong Province Medical Science Research Foundation (A2017308) (to X.C.) and by US NIH R01 grants HL072166 and HL085629 (to X.W.).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, X., Wu, J., Yang, Q. et al. Cadmium pyrithione suppresses tumor growth in vitro and in vivo through inhibition of proteasomal deubiquitinase. Biometals 31, 29–43 (2018). https://doi.org/10.1007/s10534-017-0062-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-017-0062-6