Abstract
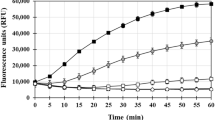
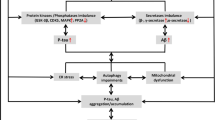
Amyloid β (Aβ) fibrils and amorphous aggregates are found in the brain of patients with Alzheimer’s disease (AD), and are implicated in the etiology of AD. The metal imbalance is also among leading causes of AD, owing to the fact that Aβ aggregation takes place in the synaptic cleft where Aβ, Cu(II) and Fe(III) are found in abnormally high concentrations. Aβ40 and Aβ42 are the main components of plaques found in afflicted brains. Coordination of Cu(II) and Fe(III) ions to Aβ peptides have been linked to Aβ aggregation and production of reactive oxygen species, two key events in the development of AD pathology. Metal chelation was proposed as a therapy for AD on the basis that it might prevent Aβ aggregation. In this work, we first examined the formation of Aβ40 and Aβ42 aggregates in the presence of metal ions, i.e. Fe(III) and Cu(II), which were detected by fluorescence spectroscopy and atomic force microscopy. Second, we studied the ability of the two chelators, ethylenediaminetetraacetic acid and 5-chloro-7-iodo-8-hydroxyquinoline (clioquinol), to investigate their effect on the availability of these metal ions to interact with Aβ and thereby their effect on Aβ accumulation. Our findings show that Fe(III), but not Cu(II), promote aggregation of both Aβ40 and Aβ42. We also found that only clioquinol decreased significantly iron ion-induced aggregation of Aβ42. The presence of ions and/or chelators also affected the morphology of Aβ aggregates.





Similar content being viewed by others
References
Cukalevski R, Yang X, Meisl G, Weininger U, Bernfur K, Frohm B, Knowles TP, Linse S (2015) The Aβ40 and Aβ42 peptides self-assemble into separate homomolecular fibrils in binary mixtures but cross-react during primary nucleation. Chem Sci 6:4215–4233
Faller P, Hureau C, Berthoumieu O (2013) Role of metal ions in the self-assembly of the Alzheimer’s amyloid Aβ peptide. Inorg Chem 52:12193–12206
Gu L, Tran J, Jiang L, Guo Z (2016) A new structural model of Alzheimer’s Aβ42 fibrils based on electron paramagnetic resonance data and Rosetta modeling. J Struct Biol 194:61–67
Horcas I, Fernández R, Gomez-Rodriguez JM, Colchero J, Gómez-Herrero J, Baro AM (2007) WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Rev Sci Instrum 78:013705
Jamasbi E, Wade DJ, Separovic F, Mohammed AH (2016) Amyloid beta (aβ) peptide and factors that play important roles in Alzheimer’s disease. Curr Med Chem 23:884–892
Jiang D, Rauda I, Han S, Chen S, Zhou F (2012) Aggregation pathways of the amyloid β(1−42) peptide depend on its colloidal stability and ordered β-sheet stacking. Langmuir 28:12711–12721
Kong X, Zhao Z, Lei X, Zhang B, Dai D, Jiang L (2015) Interaction of metal ions with the his13-his14 sequence relevant to Alzheimer’s disease. J Phys Chem A 119:3528–3534
Lim MH, Mancino AM, Hindo SS, Kochi A (2009) Effects of clioquinol on metal-triggered amyloid-β aggregation revisited. Inorg Chem 48:9596–9598
Matlack KES, Tardiff DF, Narayan P, Hamamichi S, Caldwell KA, Caldwell GA, Lindquist S (2014) Clioquinol promotes the degradation of metal-dependent amyloid-β (aβ) oligomers to restore endocytosis and ameliorate aβ toxicity. Proc Nat Acad Sci USA 111:4013–4018
Orvig C, Rodríguez-Rodríguez C, Telpoukhovskaia M (2012) The art of building multifunctional metal-binding agents from basic molecular scaffolds for the potential application in neurodegenerative diseases. Coord Chem Rev 256:2308–2332
Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V (2013) 8-Hydroxyquinolines: a review of their metal chelating properties and medicinal applications. Drug Des Devel Ther 7:1157–1178
Robert A, Liu Y, Nguyen M, Meunier B (2015) Regulation of copper and iron homeostasis by metal chelators: a possible chemotherapy for Alzheimer’s disease. Acc Chem Res 48:1332–1339
Sakono M, Zako T (2010) Amyloid oligomers: formation and toxicity of Aβ oligomers. FEBS J 277:1348–1358
Santos MA, Chand K, Chaves S (2016) Recent progress in multifunctional metal chelators as potential drugs for Alzheimer’s disease. Coord Chem Rev 327:287–303
Sastre M, Ritchie CW, Hajji N (2015) Metal ions in Alzheimer’s disease brain. JSM Alzheimer’s Dis Rel Dement 2:1014–1019
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608
Tiiman A, Krishtal J, Palumaa P, Tõugu V (2015) In vitro fibrillization of Alzheimer’s amyloid-β peptide (1–42). AIP Adv 5:092401
Wallin C, Kulkarni YS, Abelein A, Jarvet J, Liao Q, Strodel B, Olsson L, Luo J, Abrahams JP, Sholts SB, Roos PM (2016) Characterization of Mn(II) ion binding to the amyloid-β peptide in Alzheimer’s disease. J Trace Elem Med Bio 38:183–193
Wineman-Fisher V, Bloch DN, Miller Y (2016) Challenges in studying the structures of metal-amyloid oligomers related to type 2 diabetes, Parkinson’s disease, and Alzheimer’s disease. Coord Chem Rev 327:20–26
Acknowledgements
We acknowledge the support of the research council of the Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, Iran, without which this work could not be possible to start and finish. We also thank the Department of Chemistry at IASBS for their generous cooperation in providing us the opportunity to make use of their fluorescence spectroscopy facility. Also, we thank the Department of Physics at IASBS for their kind permission to use their AFM facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tahmasebinia, F., Emadi, S. Effect of metal chelators on the aggregation of beta-amyloid peptides in the presence of copper and iron. Biometals 30, 285–293 (2017). https://doi.org/10.1007/s10534-017-0005-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-017-0005-2