Abstract
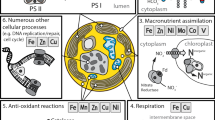
The photosynthetic picocyanobacteria and eukaryotic microorganisms that inhabit the open ocean must be able to supply iron for their photosynthetic and respiratory needs from the subnanomolar concentrations available in seawater. Neither group appears to produce siderophores, although some coastal cyanobacteria do. This is interpreted as an adaptation to the dilute oceanic environment rather than a phylogenetic constraint, since there are cases in which related taxa from different environments have the capacity to produce siderophores. Most photosynthetic marine microorganisms are presumably, however, capable of accessing iron from strong chelates since the majority of dissolved iron in seawater is complexed by organic ligands, including siderophores. Rather than direct internalization of siderophores and other iron chelates, marine organisms primarily appear to use uptake pathways that involve a reduction step to free bound iron, closely coupled with transport into the cell.


Similar content being viewed by others
References
Achilles KM, Church TM, Wilhelm SW, Luther GW, Hutchins DA (2003) Bioavailability of iron to Trichodesmium colonies in the western subtropical Atlantic Ocean. Limnol Oceanogr 48:2250–2255
Barbeau K, Rue EL, Bruland KW, Butler A (2001) Photochemical cycling of iron in the surface ocean mediated by microbial iron(III)-binding ligands. Nature 413:409–413. doi:10.1038/35096545
Boye M, van den Berg CMG (2000) Iron availability and the release of iron-complexing ligands by Emiliania huxleyi. Mar Chem 70:277–287. doi:10.1016/S0304-4203(00)00032-3
Boye M, Nishioka J, Croot PL, Laan P, Timmermans KR, de Baar HJW (2005) Major deviations of iron complexation during 22 days of a mesoscale iron enrichment in the open Southern Ocean. Mar Chem 96:257–271. doi:10.1016/j.marchem.2005.02.002
Bruland KW (1992) Complexation of cadmium by natural organic-ligands in the central North Pacific. Limnol Oceanogr 37:1008–1017
Buck KN, Bruland KW (2007) The physicochemical speciation of dissolved iron in the Bering Sea, Alaska. Limnol Oceanogr 52:1800–1808
Butler A (2005) Marine siderophores and microbial iron mobilization. Biometals 18:369–374. doi:10.1007/s10534-005-3711-0
Challis GL (2005) A widely distributed bacterial pathway for siderophore biosynthesis independent of nonribosomal peptide synthetases. ChemBioChem 6:601–611. doi:10.1002/cbic.200400283
Crosa JH, Walsh CT (2002) Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol Rev 66:223–249. doi:10.1128/MMBR.66.2.223-249.2002
Curie C, Briat JF (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54:183–206. doi:10.1146/annurev.arplant.54.031902.135018
Gledhill M, van den Berg CMG (1994) Determination of complexation of iron(III) with natural organic complexing ligands in seawater using cathodic stripping voltammetry. Mar Chem 47:41–54. doi:10.1016/0304-4203(94)90012-4
Granger J, Price NM (1999) The importance of siderophores in iron nutrition of heterotrophic marine bacteria. Limnol Oceanogr 44:541–555
Guan LL, Onuki H, Kamino K (2000) Bacterial growth stimulation with exogenous siderophore and synthetic N-acyl homoserine lactone autoinducers under iron-limited and low-nutrient conditions. Appl Environ Microbiol 66:2797–2803. doi:10.1128/AEM.66.7.2797-2803.2000
Haas H, Eisendle M, Turgeon BG (2008) Siderophores in fungal physiology and virulence. Annu Rev Phytopathol 46:149–187. doi:10.1146/annurev.phyto.45.062806.094338
Henely WJ, Yin Y (1998) Growth and photosynthesis of marine Synechococcus (Cyanophyceae) under iron stress. J Phycol 34:94–103. doi:10.1046/j.1529-8817.1998.340094.x
Honda D, Yokota A, Sugiyama J (1999) Detection of seven major evolutionary lineages in cyanobacteria based on the 16S rRNA gene sequence analysis with new sequences from five marine Synechococcus strains. J Mol Evol 48:723–739
Hudson RJM, Morel FMM (1990) Iron transport in marine phytoplankton: kinetics of cellular medium coordination reactions. Limnol Oceanogr 35:1002–1020
Hurst MP, Bruland KW (2007) An investigation into the exchange of iron and zinc between soluble, colloidal, and particulate size-fractions in shelf waters using low-abundance isotopes as tracers in shipboard incubation experiments. Mar Chem 103:211–226. doi:10.1016/j.marchem.2006.07.001
Hutchins DA, Franck VM, Brzenzinski MA, Bruland KW (1999a) Inducing phytoplankton iron limitation in iron-replete coastal waters with a strong chelating ligand. Limnol Oceanogr 44:1009–1018
Hutchins DA, Witter AE, Butler A, Luther GW (1999b) Competition among marine phytoplankton for different chelated iron species. Nature 400:858–861. doi:10.1038/23680
Ito Y, Butler A (2005) Structure of synechobactins, new siderophores of the marine cyanobacterium Synechococcus sp. PCC 7002. Limnol Oceanogr 50:1918–1923
Ito Y, Okada S, Murakami M (2001) Two structural isomeric siderophores from the freshwater cyanobacterium Anabaena cylindrica (NIES-19). Tetrahedron 57:9093–9099. doi:10.1016/S0040-4020(01)00934-6
Ito Y, Ishida K, Okada S, Murakami M (2004) The absolute stereochemistry of anachelins, siderophores from the cyanobacterium Anabaena cylindrica. Tetrahedron 60:9075–9080. doi:10.1016/j.tet.2004.07.105
Jeanjean R, Talla E, Latifi A, Havaux M, Janicki A, Zhang CC (2008) A large gene cluster encoding peptide synthetases and polyketide synthases is involved in production of siderophores and oxidative stress response in the cyanobacterium Anabaena sp. strain PCC 7120. Environ Microbiol 10:2574–2585. doi:10.1111/j.1462-2920.2008.01680.x
Johnson KS, Gordon RM, Coale KH (1997) What controls dissolved iron concentrations in the world ocean? Mar Chem 57:137–161. doi:10.1016/S0304-4203(97)00043-1
Katoh H, Hagino N, Grossman AR, Ogawa T (2001) Genes essential to iron transport in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol 183:2779–2784. doi:10.1128/JB.183.9.2779-2784.2001
Kosman DJ (2003) Molecular mechanisms of iron uptake in fungi. Mol Microbiol 47:1185–1197. doi:10.1046/j.1365-2958.2003.03368.x
Kustka AB, Shaked Y, Milligan AJ, King DW, Morel FMM (2005) Extracellular production of superoxide by marine diatoms: contrasting effects on iron redox chemistry and bioavailability. Limnol Oceanogr 50:1172–1180
Kustka AB, Allen AE, Morel FMM (2007) Sequence analysis and transcriptional regulation of iron acquisition genes in two marine diatoms. J Phycol 43:715–729. doi:10.1111/j.1529-8817.2007.00359.x
Laglera LM, van den Berg CMG (2009) Evidence for geochemical control of iron by humic substances in seawater. Limnol Oceanogr 54:610–619
Macrellis HM, Trick CG, Rue EL, Smith G, Bruland KW (2001) Collection and detection of natural iron-binding ligands from seawater. Mar Chem 76:175–187. doi:10.1016/S0304-4203(01)00061-5
Maldonado MT, Price NM (2001) Reduction and transport of organically bound iron by Thalassiosira oceanica (Bacillariophyceae). J Phycol 37:298–309. doi:10.1046/j.1529-8817.2001.037002298.x
Maldonado MT, Hughes MP, Rue EL, Wells ML (2002) The effect of Fe and Cu on growth and domoic acid production by Pseudo-nitzschia multiseries and Pseudo-nitzschia australis. Limnol Oceanogr 47:515–526
Maldonado MT, Allen AE, Chong JS, Lin K, Leus D, Karpenko N, Harris SL (2006) Copper-dependent iron transport in coastal and oceanic diatoms. Limnol Oceanogr 51:1729–1743
Martinez JS, Zhang GP, Holt PD, Jung HT, Carrano CJ, Haygood MG, Butler A (2000) Self-assembling amphiphilic siderophores from marine bacteria. Science 287:1245–1247. doi:10.1126/science.287.5456.1245
Martinez JS, Carter-Franklin JN, Mann EL, Martin JD, Haygood MG, Bulter A (2003) Structure and membrane affinity of a suite of amphiphilic siderophores produced by a marine bacterium. Proc Natl Acad Sci USA 100:3754–3759. doi:10.1073/pnas.0637444100
Mawji E, Gledhill M, Milton JA, Tarran GA, Ussher S, Thompson A, Wolff GA, Worsfold PJ, Achterberg EP (2008) Hydroxamate siderophores: occurrence and importance in the Atlantic Ocean. Environ Sci Technol 42:8675–8680. doi:10.1021/es801884r
Measures CI, Landing WM, Brown MT, Buck CS (2008) High-resolution Al and Fe data from the Atlantic Ocean CLIVAR-CO2 repeat hydrography A16 N transect: extensive linkages between atmospheric dust and upper ocean geochemistry. Global Biogeochem Cycles 22:GB1005. doi:10.1029/2007GB003042
Morel FMM, Kustka AB, Shaked Y (2008) The role of unchelated Fe in the nutrition of phytoplankton. Limnol Oceanogr 53:400–404
Nicolaisen K, Moslavac S, Samborski A, Valdebenito M, Hantke K, Maldener I, Muro-Pastor AM, Flores E, Schleiff E (2008) Alr0397 is an outer membrane transporter for the siderophore schizokinen in Anabaena sp. strain PCC 7120. J Bacteriol 190:7500–7507. doi:10.1128/JB.01062-08
Palenik B, Brahamsha B, Larmier FW, Land M, Hauser L, Chain P, Lamerdin J, Regala W, Allen EA, McCarren J, Paulsen I, Dufresne A, Partensky F, Webb EA, Waterbury J (2003) The genome of a motile marine Synechococcus. Nature 424:1037–1042. doi:10.1038/nature01943
Palenik B et al (2006) Genome sequence of Synechococcus CC9311: insights into adaptation to a coastal environment. Proc Natl Acad Sci USA 103:1355–13559. doi:10.1073/pnas.0602963103
Palenik B et al (2007) The tiny eukaryote Ostreococcus provides genomic insights into the paradox of plankton speciation. Proc Natl Acad Sci USA 104:7705–7710. doi:10.1073/pnas.0611046104
Poole K, Young L, Neshat S (1990) Enterobactin-mediated iron transport in Pseudomonas aeruginosa. J Bacteriol 172:6991–6996
Postle K, Kadner RJ (2003) Touch and go: tying TonB to transport. Mol Microbiol 49:869–882. doi:10.1046/j.1365-2958.2003.03629.x
Price NM, Morel FMM (1998) Biological cycling of iron in the ocean. In: Sigel A, Sigel H (eds) Iron transport and storage in microorganisms, plants, and animals. Metal ions in biological systems, vol 35. M. Dekker Inc, New York, pp 1–36
Raven JA (1990) Predictions of Mn and Fe use efficiencies of phototrophic growth as a function of light availability for growth and of C assimilation pathway. New Phytol 116:1–18. doi:10.1111/j.1469-8137.1990.tb00505.x
Reid RT, Live DH, Faulkner DJ, Butler A (1993) A siderophore from a marine bacterium with an exceptional ferric ion affinity constant. Nature 366:455–458. doi:10.1038/366455a0
Rose AL, Salmon TP, Lukondeh T, Neilan BA, Waite TD (2005) Use of superoxide as an electron shuttle for iron acquisition by the marine cyanobacterium Lyngbya majuscula. Environ Sci Technol 39:3708–3715. doi:10.1021/es048766c
Rue EL, Bruland KW (1995) Complexation of iron(III) by natural organic ligands in the Central North Pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method. Mar Chem 50:117–138. doi:10.1016/0304-4203(95)00031-L
Rue EL, Bruland KW (1997) The role of organic complexation on ambient iron chemistry in the equatorial Pacific Ocean and the response of a mesoscale iron addition experiment. Limnol Oceanogr 42:901–910
Rue EL, Bruland KW (2001) Domoic acid binds iron and copper: a possible role for the toxin produced by the marine diatom Pseudo-nitzschia. Mar Chem 76:127–134. doi:10.1016/S0304-4203(01)00053-6
Saito MA, Moffett JW (2001) Complexation of cobalt by natural organic ligands in the Sargasso Sea as determined by a new high-sensitivity electrochemical cobalt speciation method suitable for open ocean work. Mar Chem 75:49–68. doi:10.1016/S0304-4203(01)00025-1
Schalk IJ (2008) Metal trafficking via siderophores in Gram-negative bacteria: specificities and characteristics of the pyoverdine pathway. J Inorg Biochem 102:1159–1169. doi:10.1016/j.jinorgbio.2007.11.017
Shaked Y, Kustka AB, Morel FMM (2005) A general kinetic model for iron acquisition by eukaryotic phytoplankton. Limnol Oceanogr 50:872–882
Shi T, Falkowski PG (2008) Genome evolution in cyanobacteria: the stable core and the variable shell. Proc Natl Acad Sci USA 105:2510–2515
Simpson FB, Neilands JB (1976) Siderochromes in cyanophyceae: isolation and characterization of schizokinen from Anabaena sp. J Phycol 12:44–48
Soria-Dengg S, Horstmann U (1995) Ferrioxamines B and E as iron sources for the marine diatom Phaeodactylum tricornutum. Mar Ecol Prog Ser 127:269–277. doi:10.3354/meps127269
Stintzi A, Barnes C, Xu J, Raymond KN (2000) Microbial iron transport via a siderophore shuttle: a membrane ion transport paradigm. Proc Natl Acad Sci USA 97:10691–10696. doi:10.1073/pnas.200318797
Sunda W (2001) Bioavailability and bioaccumulation of iron in the sea. In: Turner DR, Hunter KA (eds) The biogeochemistry of iron in seawater. Wiley and Sons, West Sussex, pp 41–84
Sunda WG, Huntsman SA (1995) Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar Chem 50:189–206. doi:10.1016/0304-4203(95)00035-P
Trick CG, Andersen RJ, Gillam A, Harrison PJ (1983a) Prorocentrin: an extracellular siderophore produced by the marine dinoflagellate Prorocentrum minimum. Science 219:306–308. doi:10.1126/science.219.4582.306
Trick CG, Andersen RJ, Price NM, Gillam A, Harrison PJ (1983b) Examination of hydroxamate-siderophore production by neritic eukaryotic marine phytoplankton. Mar Biol (Berl) 75:9–17. doi:10.1007/BF00392625
Volker C, Wolf-Gladrow DA (1999) Physical limits on iron uptake mediated by siderophores or surface reductases. Mar Chem 65:227–244. doi:10.1016/S0304-4203(99)00004-3
Webb EA, Moffett JW, Waterbury JB (2001) Iron stress in open-ocean cyanobacteria (Synechococcus, Trichodesmium, and Crocosphaera spp.): identification of the IdiA protein. Appl Environ Microbiol 67:5444–5452. doi:10.1128/AEM.67.12.5444-5452.2001
Wells ML, Price NM, Bruland KW (1994) Iron limitation and the cyanobacterium Synechococcus in equatorial Pacific waters. Limnol Oceanogr 39:1481–1486
Wells ML, Trick CG, Cochlan WP, Hughes MP, Trainer VL (2005) Domoic acid: the synergy of iron, copper, and the toxicity of diatoms. Limnol Oceanogr 50:1908–1917
Wilhelm SW, Trick CG (1994) Iron-limited growth of cyanobacteria: multiple siderophore production is a common response. Limnol Oceanogr 39:1979–1984
Wu J, Luther GW (1995) Complexation of Fe(III) by natural organic ligands in the northwest Atlantic Ocean by a competitive ligand equilibration method and a kinetic approach. Mar Chem 50:159–177. doi:10.1016/0304-4203(95)00033-N
Wu J, Boyle E, Sunda WG, Wen LS (2001) Soluble and colloidal iron in the oligotrophic North Atlantic and North Pacific. Science 293:847–849. doi:10.1126/science.1059251
Acknowledgments
We would like to thank Kathy Barbeau and Yeala Shaked for providing data prior to publication and for helpful comments on the manuscript. This work has been supported by the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hopkinson, B.M., Morel, F.M.M. The role of siderophores in iron acquisition by photosynthetic marine microorganisms. Biometals 22, 659–669 (2009). https://doi.org/10.1007/s10534-009-9235-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-009-9235-2