Abstract
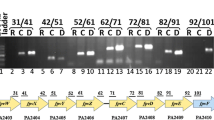
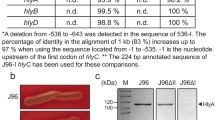
Acinetobacter baumannii is a gram-negative bacterium that causes serious infections in compromised patients. More recently, it has emerged as the causative agent of severe infections in military personnel wounded in Iraq and Afghanistan. This pathogen grows under a wide range of conditions including iron-limiting conditions imposed by natural and synthetic iron chelators. Initial studies using the type strain 19606 showed that the iron proficiency of this pathogen depends on the expression of the acinetobactin-mediated iron acquisition system. More recently, we have observed that hemin but not human hemoglobin serves as an iron source when 19606 isogenic derivatives affected in acinetobactin transport and biosynthesis were cultured under iron-limiting conditions. This finding is in agreement with the observation that the genome of the strain 17978 has a gene cluster coding for putative hemin-acquisition functions, which include genes coding for putative hemin utilization functions and a TonBExbBD energy transducing system. This system restored enterobactin biosynthesis in an E. coli ExbBD deficient strain but not when introduced into a TonB mutant. PCR and Southern blot analyses showed that this hemin-utilization gene cluster is also present in the 19606 strain. Analysis of the 17978 genome also showed that this strain harbors genes required for acinetobactin synthesis and transport as well as a gene cluster that could code for additional iron acquisition functions. This hypothesis is in agreement with the fact that the inactivation of the basD acinetobactin biosynthetic gene did not affect the growth of A. baumannii 17978 cells under iron-chelated conditions. Interestingly, this second iron uptake gene cluster is flanked by perfect inverted repeats and includes transposase genes that are expressed transcriptionally. Also interesting is the observation that this additional cluster could not be detected in the type strain 19606, an observation that suggests some significant differences in the iron uptake capacity between these two A. baumannii strains. Transposome mutagenesis of the strain 19606 resulted in the isolation of a derivative unable to grow under iron-chelated conditions. Gene mapping and protein analysis together with complementation assays showed that a protein related to SecA, which is a component of the Sec protein secretion system in a wide range of bacteria, is needed at least for the production of the BauA acinetobactin outer membrane receptor. Furthermore, this derivative was unable to use hemin as an iron source under limiting conditions. Taken together, these results indicate that A. baumannii expresses siderophore-mediated and hemin acquisition functions, although different isolates differ in their iron acquisition capacity. Unexpectedly, the ability of this pathogen to acquire iron depends on the expression of a SecA protein secretion function, which has not been associated with iron acquisition in bacteria.








Similar content being viewed by others
References
Actis LA, Tolmasky ME, Crosa LM, Crosa JH (1993) Effect of iron-limiting conditions on growth of clinical isolates of Acinetobacter baumannii. J Clin Microbiol 31:2812–2815
Alexeyev MF (1999) The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. Biotechniques 26:824–826 828
Arnow L (1937) Colorimetric determination of the components of 3,4-dihydroxyphenylalanine–tyrosine mixtures. J Biol Chem 118:531–537
Bergogne-Berezin E (2001) The increasing role of Acinetobacter species as nosocomial pathogens. Curr Infect Dis Rep 3:440–444. doi:10.1007/s11908-007-1011-2
Bergogne-Berezin E, Towner KJ (1996) Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev 9:148–165
Calhoun JH, Murray CK, Manring MM (2008) Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin Orthop Relat Res 466:1356–1362. doi:10.1007/s11999-008-0212-9
Cox GB, Gibson F, Luke RK, Newton NA, O’Brien IG, Rosenberg H (1970) Mutations affecting iron transport in Escherichia coli. J Bacteriol 104:219–226
Davis KA, Moran KA, McAllister CK, Gray PJ (2005) Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis 11:1218–1224
Di Lorenzo M et al (2003) Complete sequence of virulence plasmid pJM1 from the marine fish pathogen Vibrio anguillarum strain 775. J Bacteriol 185:5822–5830. doi:10.1128/JB.185.19.5822-5830.2003
Dijkshoorn L, Nemec A, Seifert H (2007) An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5:939–951. doi:10.1038/nrmicro1789
Dorsey CW, Beglin MS, Actis LA (2003) Detection and analysis of iron uptake components expressed by Acinetobacter baumannii clinical isolates. J Clin Microbiol 41:4188–4193. doi:10.1128/JCM.41.9.4188-4193.2003
Dorsey CW, Tomaras AP, Connerly PL, Tolmasky ME, Crosa JH, Actis LA (2004) The siderophore-mediated iron acquisition systems of Acinetobacter baumannii ATCC 19606 and Vibrio anguillarum 775 are structurally and functionally related. Microbiology 150:3657–3667. doi:10.1099/mic.0.27371-0
Echenique JR et al (1992) Characterization of a high-affinity iron transport system in Acinetobacter baumannii. J Bacteriol 174:7670–7679
Fournier PE et al (2006) Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet 2:e7. doi:10.1371/journal.pgen.0020007
Houang ET, Sormunen RT, Lai L, Chan CY, Leong AS (1998) Effect of desiccation on the ultrastructural appearances of Acinetobacter baumannii and Acinetobacter lwoffii. J Clin Pathol 51:786–788
Iacono M et al (2008) Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob Agents Chemother 52:2616–2625. doi:10.1128/AAC.01643-07
Joly-Guillou ML (2005) Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect 11:868–873. doi:10.1111/j.1469-0691.2005.01227.x
Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK (2007) The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J Clin Invest 117:877–888. doi:10.1172/JCI30783
Manting EH, Driessen AJ (2000) Escherichia coli translocase: the unravelling of a molecular machine. Mol Microbiol 37:226–238. doi:10.1046/j.1365-2958.2000.01980.x
Mihara K et al (2004) Identification and transcriptional organization of a gene cluster involved in biosynthesis and transport of acinetobactin, a siderophore produced by Acinetobacter baumannii ATCC 19606T. Microbiology 150:2587–2597. doi:10.1099/mic.0.27141-0
Mori H, Ito K (2001) The Sec protein-translocation pathway. Trends Microbiol 9:494–500. doi:10.1016/S0966-842X(01)02174-6
Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi:10.1128/CMR.00058-07
Perez F, Endimiani A, Bonomo RA (2008) Why are we afraid of Acinetobacter baumannii? Expert Rev Anti Infect Ther 6:269–271. doi:10.1586/14787210.6.3.269
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. doi:10.1016/0003-2697(87)90612-9
Smith MG et al (2007) New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev 21:601–614. doi:10.1101/gad.1510307
Tolmasky ME, Actis LA, Crosa JH (1988) Genetic analysis of the iron uptake region of the Vibrio anguillarum plasmid pJM1: molecular cloning of genetic determinants encoding a novel trans activator of siderophore biosynthesis. J Bacteriol 170:1913–1919
Tomaras AP, Dorsey CW, Edelmann RE, Actis LA (2003) Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology 149:3473–3484. doi:10.1099/mic.0.26541-0
Villegas MV, Hartstein AI (2003) Acinetobacter outbreaks, 1977–2000. Infect Control Hosp Epidemiol 24:284–295. doi:10.1086/502205
Yamamoto S, Okujo N, Sakakibara Y (1994) Isolation and structure elucidation of acinetobactin, a novel siderophore from Acinetobacter baumannii. Arch Microbiol 162:249–252
Acknowledgments
Funds from Miami University and the Public Health NIAID AI070174 grant supported the research work presented in this manuscript. We thank Dr. R. Larsen (Bowling Green State University) for providing the E. coli W3110 strain and the isogenic derivatives RA1051 and KP1344.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zimbler, D.L., Penwell, W.F., Gaddy, J.A. et al. Iron acquisition functions expressed by the human pathogen Acinetobacter baumannii . Biometals 22, 23–32 (2009). https://doi.org/10.1007/s10534-008-9202-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-008-9202-3