Abstract
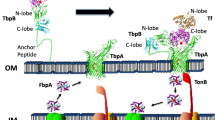
Iron is an essential nutrient for all microorganisms with a few exceptions. Microorganisms use a variety of systems to acquire iron from the surrounding environment. One such system includes production of an organic molecule known as a siderophore by many bacteria and fungi. Siderophores have the capacity to specifically chelate ferric ions. The ferricsiderophore complex is then transported into the cell via a specific receptor protein located in the outer membrane. This is an energy dependent process and is the subject of investigation in many research laboratories. The crystal structures of three outer membrane ferricsiderophore receptor proteins FepA, FhuA and FecA from Escherichia coli and two FpvA and FptA from Pseudomonas aeruginosa have recently been solved. Four of them, FhuA, FecA, FpvA and FptA have been solved in ligand-bound forms, which gave insight into the residues involved in ligand binding. The structures are similar and show the presence of similar domains; for example, all of them consist of a 22 strand-β-barrel formed by approximately 600 C-terminal residues while approximately 150 N-terminal residues fold inside the barrel to form a plug domain. The plug domain obstructs the passage through the barrel; therefore our research focuses on the mechanism through which the ferricsiderophore complex is transported across the receptor into the periplasm. There are two possibilities, one in which the plug domain is expelled into the periplasm making way for the ferricsiderophore complex and the second in which the plug domain undergoes structural rearrangement to form a channel through which the complex slides into the periplasm. Multiple alignment studies involving protein sequences of a large number of outer membrane receptor proteins that transport ferricsiderophores have identified several conserved residues. All of the conserved residues are located within the plug and barrel domain below the ligand binding site. We have substituted a number of these residues in FepA and FhuA with either alanine or glutamine resulting in substantial changes in the chemical properties of the residues. This was done to study the effect of the substitutions on the transport of ferricsiderophores. Another strategy used was to create a disulfide bond between the residues located on two adjacent β-strands of the plug domain or between the residues of the plug domain and the β-barrel in FhuA by substituting appropriate residues with cysteine. We have looked for the variants where the transport is affected without altering the binding. The data suggest a distinct role of these residues in the mechanism of transport. Our data also indicate that these transporters share a common mechanism of transport and that the plug remains within the barrel and possibly undergoes rearrangement to form a channel to transport the ferricsiderophore from the binding site to the periplasm.









Similar content being viewed by others
References
Böhm J, Lambert O, Frangakis AS, Letellier L, Baumeister W, Rigaud JL (2001) FhuA-mediated phage genome transfer into liposomes: a cryo-electron tomography study. Curr Biol 11:1168–1175
Braun V (1995) Energy-coupled transport and signal transduction through the Gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Mcrobiol Rev 16:295–307
Braun V, Braun M (2002a) Active transport of iron and siderophore antibiotics. Curr Opinion Microbiol 5:194–2001
Braun V, Braun M (2002b) Iron transport and signaling in E.coli. FEBS Lett 529:78–85
Braun V, Killmann H (1999) Bacterial solutions to the iron supply problem. Trends Biochem Sci 24:104–109
Buchanan SK, Smith BS, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J (1999) Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol 6:56–62
Chakraborty R, Lemke E, Cao Z, Klebba PE, van der Helm D (2003) Identification and mutational studies of conserved amino acids in the outer membrane receptor protein, FepA, which affect transport but not binding of ferric-enterobactin in Escherichia coli. Biometals 16:507–518
Chang C, Mooser A, Pluckthun A, Wlodawer A (2001) Crystal structure of the dimeric C-terminal domain of TonB reveals a novel fold. J Biol Chem 276:27535–27540
Chimento DP, Kadner RJ, Wiener MC (2003) The Escherichia coli outer membrane cobalamin transporter BtuB: structural analysis of calcium and substrate binding, and identification of orthologous transporters by sequence/structure conservation. J Mol Biol 322:999–1014
Chimento DP, Kadner RJ, MC Wiener (2005) Comparative structural analysis of TonB-dependent outer membrane transporters: implications for the transport cycle. Proteins: Structure, Function and Bioinformatics 59:240–251
Cobessi D, Herve C, Folschweiller N, Schalk IJ, Abdallah MA, Pattus F (2005a) The Crystal structure of the pyoverdine outer membrane receptor FpvA from P.aeruginosa at 3.6 Å resolution. J Mol Biol 347:121–134
Cobessi D, Celia H, Pattus F (2005b) Structure of ferric-pyochelin and its membrane receptor FptA from P.aeruginosa. J Mol Bio 352:893–904
Ecker DJ, Matzanke BF, Raymond KN (1986) Recognition and transport of ferric enterobactin in Escherichia coli. J Bacteriol 167:666–673
Eisenhauer HA, Shames S, Pawelek PD, Coulton JW (2005) Siderophore transport through E.coli outer membrane receptor FhuA with disulfide-tethered cork and barrel domains. J Biol Chem 280:30574–30580
Ferguson AD, Hofmann E, Coulton JW, Diederichs K, Welte W (1998) Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215–2220
Ferguson AD, Chakraborty R, Smith BS, Esser L, van der Helm D, Deisenhofer J (2002) Structural basis of gating by the outer membrane transporter FecA. Science 295:1658–1659
Franchini Massimo (2006) Hereditary iron overload: update on pathophysiology, diagnosis, and treatment. Am J Hematology 81:202–209
Ghosh A, Miller M (1996) Synthesis and In Vitro antibacterial activity of spermidine-based mixed catechol and hydroxamate containing siderophore-vancomycin conjugates. Bioorg Med Chem 4:43–48
Gudmundsdottir A, Bell PE, Lundrigan MD, Bradbeer C, Kadner RJ (1989) Point mutations in a conserved region (TonB box) of Escherichia coli outer membrane protein BtuB affect vitamin B12 transport. J Bacteriol 171:6526–6533
Guiseppi JD, Fridovich I (1982) Oxygen toxicity in Streptococcus Sanguis. J Biol Chem 257:4046–4051
Ködding J, Killig F, Polzer P, Howard SP, Diederichs K, Welte W (2005) Crystal structure of a 92-residue C-termina fragment of TonB from E.coli reveals significant conformational changes compared to structures of smaller TonB fragments. J Biol Chem 280:3022–3088
Kurisu G, Zakharov SD, Zhalnina MV, Bano S, Eroukova VY, Rokitskaya TI, Antonenko YN, Wiener MC, Cramer WA (2003) The structure of BtuB with bound colicin E3 R-domain implies a translocon. Nat Struct Biol 11:948–954
Locher KP, Rees B, Koebnik R, Mitschler A, Moulinier L, Rosenbusch JP, Moras D (1998) Trans membrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95:771–778
Lo Conte L, Chothia C, Janin J (1999) The atomic structure of protein–protein recognition sites. J Mol Biol 285:2177–2198
Lundrigan MD, Kadner RJ (1986) Nucleotide sequence of the gene for the ferrienterochelin receptor FepA in E.coli. Homology among outer membrane receptors that interact with TonB. J Biol Chem 261:10797–10801
Miller MJ, Malouin F (1993) Microbial iron chelators as drug delivery agents: the rational design and synthesis of siderophore-drug conjugates. Acc Chem Res 26:241–249
Neilands JB (1981) Microbial iron compounds. Ann Rev Biochem 50:715–731
Pawelek PD, Croteau N, Ng-Thow-Hing C, Khursigara CM, Moiseeva N, Allaire M, Coulton JW (2006) Structure of TonB in complex with FhuA, E.coli outer membrane receptor. Science 312:1399–1402
Peacock RS, Weljie AM, Howard SP, Price FD, Vogel HJ (2005) The solution structure of the C-terminal domain of TonB and interactions studies with TonB box peptides. J Mol Biol 345:1185–1197
Plancon L, Janmot C, Ie Maire M, Desmadril M, Bonhivers M, Letellier L, Boulanger P (2002) Characterization of high affinity complex between the bacterial membrane protein FhuA and the phage T5 protein pb5. J Mol Biol 318:557–569
Postle K (1993) TonB protein and energy transduction between membranes. J Bioenerg Biomembr 25:591–601
Raymond K, Dertz RE, Kim SS (2003) Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci 100:3584–3588
Sauer M, Hantke K, Braun V (1990) Sequence of the fhuE outer-membrane receptor gene of Escherichia coli K12 and properties of mutants. Mol Microbiol 4:427–437
Schryvers AB, Stojiljkovic I (1999) Iron acquisition systems in the pathogenic Neisseria. Mol Microbiol 32:1117–1123
Shultis DD, Purdy MD, Banchs CN, Wiener MC (2006) Outermembrane active transport: Structure of the BtuB:TonB complex. Science 312:1396–1399
Storey EP, Boghozian R, Little JL, Lowman DW, Chakraborty R (2006) Characterization of ‘Schizokinen’ a dihydroxamate-type siderophore produced by Rhizobium leguminosarum IARI 917. BioMetals 19:637–649
Tsai CJ, Lin SL, Wolfson HJ, Nussinov R (1997) Studies of protein-protein interfaces: a statistical analysis of the hydrophobic effect. Protein Sci 6:53–64
Weinberg ED (1984) Iron withholding: a defense against infection and neoplasia. Physiol Rev 64:65–100
van der Helm D, Baker JR, Eng-Wilmot DL, Hossain MB, Loghry RA (1980) Crystal structure of ferrichrome and a comparison with the structure of ferrichrome A. J Am Chem Soc 102:4224–4231
van der Helm D, Jalal MAF, Hossain MB (1987) The crystal structures, conformations and configurations of siderophores. In: Winkelmann G van der Helm D Neilands JB (eds) In iron transport in microbes, plants and animals. Verlag Chemie, Weinheim, FRG, pp 135–165
van der Helm D, Chakraborty R (2001) Structures of siderophore receptors. In: Winkelman G (ed) Microbial transport systems. Wiley-VCH, Weinheim, Germany, pp 261–288
van der Helm D, Chakraborty R, Ferguson AD, Smith BS, Lothar E, Deisenhofer J (2002) Bipartite gating in the outer membrane protein FecA. Biochemical Transactions, Biochem Soc Trans 30:708–710
van der Helm D (2004) Structure of outer membrane receptor proteins. In: Crosa JH, Mey AR, Payne SM (eds) Iron transport in bacteria, ASM press, Washington, DC, USA, pp 51–65
Wiener MC (2005) TonB-dependent outer membrane transport: going for Baroque? Curr opinion in Struct Biol 15:394–400
Winkelmann G (ed) 1991 Handbook of microbial iron chelates. CRC Press, Boca Raton, FL
Acknowledgments
We thank Bert Lampson, Allan Forsman and Michael Gallagher for critical reading of the manuscript, Ralph Coffman, Lisa Gallagher and Lanisha Howze for technical assistance. This study was supported by Department of Health Sciences, ETSU and grants from NIH, GM21822 (DvdH) and GM069367 (RC) and RDC 03-008M (RC) from ETSU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chakraborty, R., Storey, E. & van der Helm, D. Molecular mechanism of ferricsiderophore passage through the outer membrane receptor proteins of Escherichia coli . Biometals 20, 263–274 (2007). https://doi.org/10.1007/s10534-006-9060-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-006-9060-9