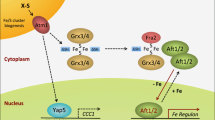
Abstract
Schizosaccharomyces pombe has acquisition processes for iron, an essential nutrient. One pathway consists to produce, excrete, and capture siderophore–iron complexes. A second pathway requires enzymatic reduction of ferric iron at the cell surface prior to uptake by a permease–oxidase complex. Genes encoding proteins involved in iron assimilation are transcriptionally regulated as a function of iron availability. Under high iron conditions, the GATA-type regulator Fep1 represses the expression of iron uptake genes. The repressor function of Fep1 requires the presence of the Tup11 or Tup12 transcriptional co-repressor. Under low iron conditions, two regulatory mechanisms occur. First, the iron transport genes are highly induced. Second, there is a transcription factor cascade implicating the heteromeric CCAAT-binding complex that turns off a set of genes encoding iron-utilizing proteins, presumably to avoid a futile expenditure of energy in producing iron-using proteins that lack the necessary cofactor to function. Thus, collectively, these regulatory responses to variations in iron concentrations ensure that iron is present within cells for essential biochemical reactions, yet prevent the accumulation of iron or iron-using proteins to deleterious levels.


Similar content being viewed by others
Notes
B. Pelletier, A. Mercier, and S. Labbé, unpublished data.
M. Durand, B. Pelletier, M. Bisaillon, and S. Labbé, unpublished data.
A. Mercier and S. Labbé, unpublished data.
In this review, we used the nomenclature for Schizosaccharomyces pombe and Saccharomyces cerevisiae genes and proteins. S. pombe wild-type genes are in regular text, italicized, with a superscripted “+” at the end (e.g., fep1 +). S. cerevisiae wild-type genes are capitalized and italicized (e.g., AFT1). S. pombe and S. cerevisiae protein nomenclature is the same and is indicated with a capital letter at the beginning followed by regular letters (e.g., Aft1, Fep1).
Abbreviations
- AD:
-
Activation domain
- K d,app :
-
Apparent dissociation constant
- bp:
-
Base pair(s)
- BPS:
-
Bathophenanthrolinedisulfonic acid
- DBD:
-
DNA-binding domain
- Dip:
-
2,2’ Dipyridyl
- Fep1:
-
Fe protein 1
- ORF:
-
Open reading frame
- ZF1:
-
N-terminal zinc finger
- ZF2:
-
C-terminal zinc finger
References
An Z, Mei B, Yuan WM, Leong SA (1997a) The distal GATA sequences of the sid1 promoter of Ustilago maydis mediate iron repression of siderophore production and interact directly with Urbs1, a GATA family transcription factor. EMBO J 16:1742–1750
An Z, Zhao Q, McEvoy J, Yuan WM, Markley JL, Leong SA (1997b) The second finger of Urbs1 is required for iron-mediated repression of sid1 in Ustilago maydis. Proc Natl Acad Sci USA 94:5882–5887
Askwith C, Eide D, Van Ho A, Bernard PS, Li L, Davis-Kaplan S, Sipe DM, Kaplan J (1994) The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76:403–410
Askwith C, Kaplan J (1997) An oxidase–permease-based iron transport system in Schizosaccharomyces pombe and its expression in Saccharomyces cerevisiae. J Biol Chem 272:401–405
Balasubramanian B, Lowry CV, Zitomer RS (1993) The Rox1 repressor of the Saccharomyces cerevisiae hypoxic genes is a specific DNA-binding protein with a high-mobility-group motif. Mol Cell Biol 13:6071–6078
Blackburn NJ, Ralle M, Hassett R, Kosman DJ (2000) Spectroscopic analysis of the trinuclear cluster in the Fet3 protein from yeast, a multinuclear copper oxidase. Biochemistry 39:2316–2324
Blaiseau PL, Lesuisse E, Camadro JM (2001) Aft2p, a novel iron-regulated transcription activator that modulates, with Aft1p, intracellular iron use and resistance to oxidative stress in yeast. J Biol Chem 276:34221–34226
Crisp RJ, Adkins EM, Kimmel E, Kaplan J (2006) Recruitment of Tup1p and Cti6p regulates heme-deficient expression of Aft1p target genes. EMBO J 25:512–521
Dancis A, Klausner RD, Hinnebusch AG, Barriocanal JG (1990) Genetic evidence that ferric reductase is required for iron uptake in Saccharomyces cerevisiae. Mol Cell Biol 10:2294–2301
Dancis A, Roman DG, Anderson GJ, Hinnebusch AG, Klausner RD (1992) Ferric reductase of Saccharomyces cerevisiae: molecular characterization, role in iron uptake, and transcriptional control by iron. Proc Natl Acad Sci USA 89:3869–3873
De Silva DM, Askwith CC, Eide D, Kaplan J (1995) The FET3 gene product required for high affinity iron transport in yeast is a cell surface ferroxidase. J Biol Chem 270:1098–1101
Eisendle M, Oberegger H, Zadra I, Haas H (2003) The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding l-ornithine N 5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol Microbiol 49:359–375
Fagerstrom-Billai F, Wright AP (2005) Functional comparison of the Tup11 and Tup12 transcriptional corepressors in fission yeast. Mol Cell Biol 25:716–727
Felice MR, De Domenico I, Li L, Ward DM, Bartok B, Musci G, Kaplan J (2005) Post-transcriptional regulation of the yeast high affinity iron transport system. J Biol Chem 280:22181–22190
Finegold AA, Shatwell KP, Segal AW, Klausner RD, Dancis A (1996) Intramembrane bis-heme motif for transmembrane electron transport conserved in a yeast iron reductase and the human NADPH oxidase. J Biol Chem 271:31021–31024
Forsburg SL, Guarente L (1989) Identification and characterization of HAP4: a third component of the CCAAT-bound HAP2/HAP3 heteromer. Genes Dev 3:1166–1178
Garnier J, Gibrat JF, Robson B (1996) GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzymol 266:540–553
Georgatsou E, Alexandraki D (1994) Two distinctly regulated genes are required for ferric reduction, the first step of iron uptake in Saccharomyces cerevisiae. Mol Cell Biol 14:3065–3073
Greenall A, Hadcroft AP, Malakasi P, Jones N, Morgan BA, Hoffman CS, Whitehall SK (2002) Role of fission yeast Tup1-like repressors and Prr1 transcription factor in response to salt stress. Mol Biol Cell 13:2977–2989
Haas H (2003) Molecular genetics of fungal siderophore biosynthesis and uptake: the role of siderophores in iron uptake and storage. Appl Microbiol Biotechnol 62:316–330
Haas H, Zadra I, Stoffler G, Angermayr K (1999) The Aspergillus nidulans GATA factor SREA is involved in regulation of siderophore biosynthesis and control of iron uptake. J Biol Chem 274:4613–4619
Halliwell B, Gutteridge JM (1992) Biologically relevant metal ion-dependent hydroxyl radical generation. FEBS Lett 307:108–112
Harrison KA, Marzluf GA (2002) Characterization of DNA binding and the cysteine rich region of SRE, a GATA factor in Neurospora crassa involved in siderophore synthesis. Biochemistry 41:15288–15295
Hassett RF, Yuan DS, Kosman DJ (1998) Spectral and kinetic properties of the Fet3 protein from Saccharomyces cerevisiae, a multinuclear copper ferroxidase enzyme. J Biol Chem 273:23274–23282
Hentze MW, Muckenthaler MU, Andrews NC (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117:285–297
Heymann P, Ernst JF, Winkelmann G (1999) Identification of a fungal triacetylfusarinine C siderophore transport gene (TAF1) in Saccharomyces cerevisiae as a member of the major facilitator superfamily. Biometals 12:301–306
Heymann P, Ernst JF, Winkelmann G (2000a) A gene of the major facilitator superfamily encodes a transporter for enterobactin (Enb1p) in Saccharomyces cerevisiae. Biometals 13:65–72
Heymann P, Ernst JF, Winkelmann G (2000b) Identification and substrate specificity of a ferrichrome-type siderophore transporter (Arn1p) in Saccharomyces cerevisiae. FEMS Microbiol Lett 186:221–227
Janoo RT, Neely LA, Braun BR, Whitehall SK, Hoffman CS (2001) Transcriptional regulators of the Schizosaccharomyces pombe fbp1 gene include two redundant Tup1p-like corepressors and the CCAAT-binding factor activation complex. Genetics 157:1205–1215
Kaplan J, McVey Ward D, Crisp RJ, Philpott CC (2006) Iron-dependent metabolic remodeling in S. cerevisiae. Biochim Biophys Acta 1763:646–651
Knight SAB, Lesuisse E, Stearman R, Klausner RD, Dancis A (2002) Reductive iron uptake by Candida albicans: role of copper, iron and the TUP1 regulator. Microbiology 148:29–40
Komachi K, Johnson AD (1997) Residues in the WD repeats of Tup1 required for interaction with alpha2. Mol Cell Biol 17:6023–6028
Kosman DJ (2003) Molecular mechanisms of iron uptake in fungi. Mol Microbiol 47:1185–1197
Labbé S, Peña MMO, Fernandes AR, Thiele DJ (1999) A copper-sensing transcription factor regulates iron uptake genes in Schizosaccharomyces pombe. J Biol Chem 274:36252–36260
Lan CY, Rodarte G, Murillo LA, Jones T, Davis RW, Dungan J, Newport G, Agabian N (2004) Regulatory networks affected by iron availability in Candida albicans. Mol Microbiol 53:1451–1469
Lesuisse E, Crichton RR, Labbé P (1990) Iron-reductases in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 1038:253–259
Lesuisse E, Labbé P (1989) Reductive and non-reductive mechanisms of iron assimilation by the yeast Saccharomyces cerevisiae. J Gen Microbiol 135:257–263
Lesuisse E, Simon-Casteras M, Labbé P (1998) Siderophore-mediated iron uptake in Saccharomyces cerevisiae: the SIT1 gene encodes a ferrioxamine B permease that belongs to the major facilitator superfamily. Microbiology 144:3455–3462
Lupas A, Van Dyke M, Stock J (1991) Predicting coiled coils from protein sequences. Science 252:1162–1164
Maity SN, de Crombrugghe B (1998) Role of the CCAAT-binding protein CBF/NF-Y in transcription. Trends Biochem Sci 23:174–178
Mantovani R (1998) A survey of 178 NF-Y binding CCAAT boxes. Nucleic Acids Res 26:1135–1143
Massé E, Arguin M (2005) Ironing out the problem: new mechanisms of iron homeostasis. Trends Biochem Sci 30:462–468
Massé E, Escorcia FE, Gottesman S (2003) Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev 17:2374–2383
Massé E, Gottesman S (2002) A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci USA 99:4620–4625
Massé E, Vanderpool CK, Gottesman S (2005) Effect of RyhB small RNA on global iron use in Escherichia coli. J Bacteriol 187:6962–6971
Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O (2003) Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299:906–909
McNabb DS, Pinto I (2005) Assembly of the Hap2p/Hap3p/Hap4p/Hap5p-DNA complex in Saccharomyces cerevisiae. Eukaryot Cell 4:1829–1839
McNabb DS, Tseng KA, Guarente L (1997) The Saccharomyces cerevisiae Hap5p homolog from fission yeast reveals two conserved domains that are essential for assembly of heterotetrameric CCAAT-binding factor. Mol Cell Biol 17:7008–7018
McNabb DS, Xing Y, Guarente L (1995) Cloning of yeast HAP5: a novel subunit of a heterotrimeric complex required for CCAAT binding. Genes Dev 9:47–58
Mercier A, Pelletier B, Labbé S (2006) A transcription factor cascade involving Fep1 and the CCAAT-binding factor Php4 regulates gene expression in response to iron deficiency in fission yeast Schizosaccharomyces pombe. Eukaryot Cell 5:1866–1881
Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270:26723–26726
Oberegger H, Schoeser M, Zadra I, Abt B, Haas H (2001) SREA is involved in regulation of siderophore biosynthesis, utilization and uptake in Aspergillus nidulans. Mol Microbiol 41:1077–1089
Oberegger H, Zadra I, Schoeser M, Abt B, Parson W, Haas H (2002) Identification of members of the Aspergillus nidulans SREA regulon: genes involved in siderophore biosynthesis and utilization. Biochem Soc Trans 30:781–783
Olesen JT, Fikes JD, Guarente L (1991) The Schizosaccharomyces pombe homolog of Saccharomyces cerevisiae HAP2 reveals selective and stringent conservation of the small essential core protein domain. Mol Cell Biol 11:611–619
Olesen JT, Guarente L (1990) The HAP2 subunit of yeast CCAAT transcriptional activator contains adjacent domains for subunit association and DNA recognition: model for the HAP2/3/4 complex. Genes Dev 4:1714–1729
Pelletier B, Beaudoin J, Mukai Y, Labbé S (2002) Fep1, an iron sensor regulating iron transporter gene expression in Schizosaccharomyces pombe. J Biol Chem 277:22950–22958
Pelletier B, Beaudoin J, Philpott CC, Labbé S (2003) Fep1 represses expression of the fission yeast Schizosaccharomyces pombe siderophore–iron transport system. Nucleic Acids Res 31:4332–4344
Pelletier B, Trott A, Morano KA, Labbé S (2005) Functional characterization of the iron-regulatory transcription factor Fep1 from Schizosaccharomyces pombe. J Biol Chem 280:25146–25161
Philpott CC (2006) Iron uptake in fungi: a system for every source. Biochim Biophys Acta 1763:636–645
Pinkham JL, Guarente L (1985) Cloning and molecular analysis of the HAP2 locus: a global regulator of respiratory genes in Saccharomyces cerevisiae. Mol Cell Biol 5:3410–3416
Puig S, Askeland E, Thiele DJ (2005) Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 120:99–110
Ramanan N, Wang Y (2000) A high-affinity iron permease essential for Candida albicans virulence. Science 288:1062–1064
Roman DG, Dancis A, Anderson GJ, Klausner RD (1993) The fission yeast ferric reductase gene frp1 + is required for ferric iron uptake and encodes a protein that is homologous to the gp91-phox subunit of the human NADPH phagocyte oxidoreductase. Mol Cell Biol 13:4342–4350
Rutherford JC, Jaron S, Ray E, Brown PO, Winge DR (2001) A second iron-regulatory system in yeast independent of Aft1p. Proc Natl Acad Sci USA 98:14322–14327
Rutherford JC, Jaron S, Winge DR (2003) Aft1p and Aft2p mediate iron-responsive gene expression in yeast through related promoter elements. J Biol Chem 278:27636–27643
Schrettl M, Winkelmann G, Haas H (2004) Ferrichrome in Schizosaccharomyces pombe–an iron transport and iron storage compound. Biometals 17:647–654
Segal AW, West I, Wientjes F, Nugent JH, Chavan AJ, Haley B, Garcia RC, Rosen H, Scrace G (1992) Cytochrome b-245 is a flavocytochrome containing FAD and the NADPH-binding site of the microbicidal oxidase of phagocytes. Biochem J 284:781–788
Severance S, Chakraborty S, Kosman DJ (2004) The Ftr1p iron permease in the yeast plasma membrane: orientation, topology and structure-function relationships. Biochem J 380:487–496
Smith RL, Johnson AD (2000) Turning genes off by Ssn6-Tup1: a conserved system of transcriptional repression in eukaryotes. Trends Biochem Sci 25:325–330
Solomon EI, Sundaram UM, Machonkin TE (1996) Multicopper oxidases and oxygenases. Chem Rev 96:2563–2606
Spizzo T, Byersdorfer C, Duesterhoeft S, Eide D (1997) The yeast FET5 gene encodes a FET3-related multicopper oxidase implicated in iron transport. Mol Gen Genet 256:547–556
Stearman R, Yuan DS, Yamaguchi-Iwai Y, Klausner RD, Dancis A (1996) A permease–oxidase complex involved in high-affinity iron uptake in yeast. Science 271:1552–1557
Stebbins JL, Triezenberg SJ (2004) Identification, mutational analysis, and coactivator requirements of two distinct transcriptional activation domains of the Saccharomyces cerevisiae Hap4 protein. Eukaryot Cell 3:339–347
Sybirna K, Guiard B, Li YF, Bao WG, Bolotin-Fukuhara M, Delahodde A (2005) A new Hansenula polymorpha HAP4 homologue which contains only the N-terminal conserved domain of the protein is fully functional in Saccharomyces cerevisiae. Curr Genet 47:172–181
Taylor AB, Stoj CS, Ziegler L, Kosman DJ, Hart PJ (2005) The copper-iron connection in biology: structure of the metallo-oxidase Fet3p. Proc Natl Acad Sci USA 102:15459–15464
Treitel MA, Carlson M (1995) Repression by SSN6-TUP1 is directed by MIG1, a repressor/activator protein. Proc Natl Acad Sci USA 92:3132–3136
Urbanowski JL, Piper RC (1999) The iron transporter Fth1p forms a complex with the Fet5 iron oxidase and resides on the vacuolar membrane. J Biol Chem 274:38061–38070
Varanasi US, Klis M, Mikesell PB, Trumbly RJ (1996) The Cyc8 (Ssn6)-Tup1 corepressor complex is composed of one Cyc8 and four Tup1 subunits. Mol Cell Biol 16:6707–6714
Wilderman PJ, Sowa NA, FitzGerald DJ, FitzGerald PC, Gottesman S, Ochsner UA, Vasil ML (2004) Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc Natl Acad Sci USA 101:9792–9797
Winkelmann G (2002) Microbial siderophore-mediated transport. Biochem Soc Trans 30:691–696
Wolf E, Kim PS, Berger B (1997) MultiCoil: a program for predicting two- and three-stranded coiled coils. Protein Sci 6:1179–1189
Xing Y, Fikes JD, Guarente L (1993) Mutations in yeast HAP2/HAP3 define a hybrid CCAAT box binding domain. EMBO J 12:4647–4655
Yamaguchi-Iwai Y, Dancis A, Klausner RD (1995) AFT1: a mediator of iron regulated transcriptional control in Saccharomyces cerevisiae. EMBO J 14:1231–1239
Yamaguchi-Iwai Y, Stearman R, Dancis A, Klausner RD (1996) Iron-regulated DNA binding by the AFT1 protein controls the iron regulon in yeast. EMBO J 15:3377–3384
Yuan WM, Gentil GD, Budde AD, Leong SA (2001) Characterization of the Ustilago maydis sid2 gene, encoding a multidomain peptide synthetase in the ferrichrome biosynthetic gene cluster. J Bacteriol 183:4040–4051
Yun CW, Ferea T, Rashford J, Ardon O, Brown PO, Botstein D, Kaplan J, Philpott CC (2000a) Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. Evidence for two pathways of iron uptake. J Biol Chem 275:10709–10715
Yun CW, Tiedeman JS, Moore RE, Philpott CC (2000b) Siderophore-iron uptake in Saccharomyces cerevisiae. Identification of ferrichrome and fusarinine transporters. J Biol Chem 275:16354–16359
Zhou LW, Haas H, Marzluf GA (1998) Isolation and characterization of a new gene, sre, which encodes a GATA-type regulatory protein that controls iron transport in Neurospora crassa. Mol Gen Genet 259:532–540
Znaidi S, Pelletier B, Mukai Y, Labbé S (2004) The Schizosaccharomyces pombe corepressor Tup11 interacts with the iron-responsive transcription factor Fep1. J Biol Chem 279:9462–9474
Acknowledgments
We are grateful to Éric Massé for critically reading the manuscript and for his valuable comments. We thank our colleagues of the Labbé laboratory for their contributions. Research in the Labbé laboratory on iron homeostasis in fission yeast is funded by the Natural Sciences and Engineering Research Council of Canada (NSERC Grant 238238-01). A. M. and B. P. are recipients of studentships from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Fonds de la Recherche en Santé du Québec (FRSQ), respectively. S. L. is a Scholar from the FRSQ.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Labbé, S., Pelletier, B. & Mercier, A. Iron homeostasis in the fission yeast Schizosaccharomyces pombe . Biometals 20, 523–537 (2007). https://doi.org/10.1007/s10534-006-9056-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-006-9056-5