Abstract
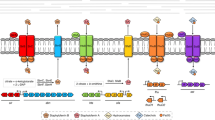
The bacterial pathogen Staphylococcus aureus is responsible for a significant amount of human morbidity and mortality, and the ability of S. aureus to cause disease is absolutely dependent on the acquisition of iron from the host. The most abundant iron source to invading staphylococci is in the form of the porphyrin heme. S. aureus is capable of acquiring nutrient iron from heme and hemoproteins via two heme-acquisition systems, the iron-regulated surface determinant system (Isd) and the heme transport system (Hts). Heme acquisition through these systems is involved in staphylococcal pathogenesis suggesting that the intracellular fate of heme plays a significant role in the infectious process. The valuable heme molecule presents a paradox to invading bacteria because although heme is an abundant source of nutrient iron, the extreme reactivity of heme makes it toxic at high concentrations. Therefore, bacteria must regulate the levels of intracellular heme to avoid toxicity. Although the molecular mechanisms responsible for staphylococcal heme acquisition are beginning to emerge, the mechanisms by which S. aureus regulate intracellular heme homeostasis are largely unknown. In this review we describe three potential fates of host-derived heme acquired by S. aureus during infection: (i) degradation for use as a nutrient iron source, (ii) incorporation into bacterial heme-binding proteins for use as an enzyme cofactor, or (iii) efflux through a dedicated ABC-type transport system. We hypothesize that the ultimate fate of exogenously acquired heme in S. aureus is dependent upon the intracellular and extracellular availability of both iron and heme.
Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bateman A, Birney E, Cerruti L et al (2002) The Pfam protein families database. Nucleic Acids Res 30: 276–280
Bibb LA, King ND, Kunkle CA, Schmitt MP (2005) Analysis of a heme-dependent signal transduction system in Corynebacterium diphtheriae: deletion of the chrAS genes results in heme sensitivity and diminished heme-dependent activation of the hmuO promoter. Infect Immun 73:7406–7412
Bozja J, Yi K, Shafer WM, Stojiljkovic I (2004) Porphyrin-based compounds exert antibacterial action against the sexually transmitted pathogens Neisseria gonorrhoeae and Haemophilus ducreyi. Int J Antimicrob Agents 24:578–584
Braun V (2001) Iron uptake mechanisms and their regulation in pathogenic bacteria. Int J Med Microbiol 291:67–79
Bullen JJ, Griffiths E (1999) Iron and infection: molecular, physiological and clinical aspects. John Wiley and Sons, New York
Chen CJ, Tobiason DM, Thomas CE, Shafer WM, Seifert HS, Sparling PF (2004) A mutant form of the Neisseria gonorrhoeae pilus secretin protein PilQ allows increased entry of heme and antimicrobial compounds. J Bacteriol 186:730–739
Clements MO, Watson SP, Poole RK, Foster SJ (1999) CtaA of Staphylococcus aureus is required for starvation survival, recovery, and cytochrome biosynthesis. J Bacteriol 181:501–507
Crichton R (2001) Inorganic biochemistry of Iron metabolism: from molecular mechanisms to clinical consequences. John Wiley & Sons, Ltd, West Sussex, England
Drazek ES, Hammack CA, Schmitt MP (2000) Corynebacterium diphtheriae genes required for acquisition of iron from haemin and haemoglobin are homologous to ABC haemin transporters. Mol Microbiol 36:68–84
Dryla A, Gelbmann D, Von Gabain A, Nagy E (2003) Identification of a novel iron regulated staphylococcal surface protein with haptoglobin-haemoglobin binding activity. Mol Microbiol 49:37–53
Everse J, Hsia N (1997) The toxicities of native and modified hemoglobins. Free Radic Biol Med 22:1075–1099
Foster TJ (2005) Immune evasion by staphylococci. Nat Rev Microbiol 3:948–958
Frankenberg-Dinkel N (2004) Bacterial heme oxygenases. Antioxid Redox Signal 6:825–834
Friedman DB, Whitwell C, Stauff, D, Pishchany G, Skaar, EP (2006) Staphylococcus aureus redirects central metabolism to increase iron availability. PLOS Pathogens Submitted
Genco CA, Dixon DW (2001) Emerging strategies in microbial haem capture. Mol Microbiol 39:1–11
Heinrichs DE (2004) Staphylococcus, Streptococcus, and Bacillus. In: Jorge H, Crosa ARM, Shelley M, Payne (ed) Iron transport in bacteria. Washington, DC, A.S.M. Press, 387–401
Kirby AE, Metzger DJ, Murphy ER, Connell TD (2001) Heme utilization in Bordetella avium is regulated by RhuI, a heme-responsive extracytoplasmic function sigma factor. Infect Immun 69:6951–6961
Klapper DG, Cuchens MA, Clem LW (1972) Induction of hemopexin by streptococcal cells in aged mice. Lab Invest 26:731–734
Ladan H, Nitzan Y, Malik Z (1993) The antibacterial activity of haemin compared with cobalt, zinc and magnesium protoporphyrin and its effect on potassium loss and ultrastructure of Staphylococcus aureus. FEMS Microbiol Lett 112:173–177
Lansky IB, Lukat-Rodgers GS, Block D, Rodgers KR, Ratliff M, Wilks A (2006) The cytoplasmic heme-binding protein (PhuS) from the heme uptake system of Pseudomonas aeruginosa is an intracellular heme-trafficking protein to the delta-regioselective heme oxygenase. J Biol Chem 281:13652–13662
Mack J, Vermeiren C, Heinrichs DE, Stillman MJ (2004) In vivo heme scavenging by Staphylococcus aureus IsdC and IsdE proteins. Biochem Biophys Res Commun 320:781–788
Mazmanian SK, Skaar EP, Gaspar AH et al (2003) Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299:906–909
Orenstein A, Klein D, Kopolovic J et al (1997) The use of␣porphyrins for eradication of Staphylococcus aureus in burn wound infections. FEMS Immunol Med Microbiol 19:307–314
Paiva-Silva GO, Cruz-Oliveira C, Nakayasu ES et al (2006) A heme-degradation pathway in a blood-sucking insect. Proc Natl Acad Sci USA 103:8030–8035
Rasmussen AW, Alexander HL, Perkins-Balding D, Shafer WM, Stojiljkovic I (2005) Resistance of Neisseria meningitidis to the toxic effects of heme iron and other hydrophobic agents requires expression of ght. J Bacteriol 187:5214–5223
Ratliff M, Zhu W, Deshmukh R, Wilks A, Stojiljkovic I (2001) Homologues of neisserial heme oxygenase in gram-negative bacteria: degradation of heme by the product of the pigA gene of Pseudomonas aeruginosa. J Bacteriol 183:6394–6403
Schiott T, Throne-Holst M, Hederstedt L (1997) Bacillus subtilis CcdA-defective mutants are blocked in a late step of cytochrome c biogenesis. J Bacteriol 179: 4523–4529
Schmitt MP (1997a) Transcription of the Corynebacterium diphtheriae hmuO gene is regulated by iron and heme. Infect Immun 65:4634–4641
Schmitt MP (1997b) Utilization of host iron sources by Corynebacterium diphtheriae: identification of a gene␣whose product is homologous to eukaryotic heme oxygenases and is required for acquisition of iron from heme and hemoglobin. J Bacteriol 179: 838–845
Schmitt MP (1999) Identification of a two-component signal transduction system from Corynebacterium diphtheriae that activates gene expression in response to the presence of heme and hemoglobin. J Bacteriol 181:5330–5340
Schuller DJ, Wilks A, Ortiz de Montellano PR, Poulos TL (1999) Crystal structure of human heme oxygenase-1. Nat Struct Biol 6:860–867
Sciara G, Kendrew SG, Miele AE et al (2003) The structure of ActVA-Orf6, a novel type of monooxygenase involved in actinorhodin biosynthesis. EMBO J 22:205–215
Skaar EP, Gaspar AH, Schneewind O (2004a) IsdG and IsdI, heme-degrading enzymes in the cytoplasm of Staphylococcus aureus. J Biol Chem 279:436–443
Skaar EP, Gaspar AH, Schneewind O (2006) Bacillus anthracis IsdG, a heme-degrading monooxygenase. J␣Bacteriol 188:1071–1080
Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O (2004b) Iron-source preference of Staphylococcus aureus infections. Science 305:1626–1628
Skaar EP, Schneewind O (2004) Iron-regulated surface determinants (Isd) of Staphylococcus aureus: stealing iron from heme. Microbes Infect 6:390–397
Stojiljkovic I, Hantke K (1994) Transport of haemin across the cytoplasmic membrane through a haemin-specific periplasmic binding-protein-dependent transport system in Yersinia enterocolitica. Mol Microbiol 13:719–732
Stojiljkovic I, Kumar V, Srinivasan N (1999) Non-iron metalloporphyrins: potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol Microbiol 31:429–442
Stojiljkovic I, Perkins-Balding D (2002) Processing of heme and heme-containing proteins by bacteria. DNA Cell Biol 21:281–295
Suits MD, Pal GP, Nakatsu K, Matte A, Cygler M, Jia Z (2005) Identification of an Escherichia coli O157:H7 heme oxygenase with tandem functional repeats. Proc Natl Acad Sci USA 102:16955–16960
Svensson B, Hederstedt L (1994) Bacillus subtilis CtaA is a heme-containing membrane protein involved in heme A biosynthesis. J Bacteriol 176:6663–6671
Thompson JM, Jones HA, Perry RD (1999) Molecular characterization of the hemin uptake locus (hmu) from Yersinia pestis and analysis of hmu mutants for hemin and hemoprotein utilization. Infect Immun 67:3879–3892
Thony-Meyer L (1997) Biogenesis of respiratory cytochromes in bacteria. Microbiol Mol Biol Rev 61: 337–376
Todd B (2006) Beyond MRSA: VISA and VRSA: what will ward off these pathogens in health care facilities? Am J Nurs 106:28–30
Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP (2006) Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol 188:8421–8429.
Vanderpool CK, Armstrong SK (2003) Heme-responsive transcriptional activation of Bordetella bhu genes. J␣Bacteriol 185:909–917
von Eiff C, Heilmann C, Proctor RA, Woltz C, Peters G, Gotz F (1997) A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J Bacteriol 179:4706–4712
von Wachenfeldt C, Hederstedt L (1992) Molecular biology of Bacillus subtilis cytochromes. FEMS Microbiol Lett 79:91–100
Voyich JM, Braughton KR, Sturdevant DE, et al (2005) Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J␣Immunol 175:3907–3919
Wandersman C, Stojiljkovic I (2000) Bacterial heme sources: the role of heme, hemoprotein receptors and hemophores. Curr Opin Microbiol 3:215–220
Weems JJ Jr (2001) The many faces of Staphylococcus aureus infection. Recognizing and managing its life-threatening manifestations. Postgrad Med 110:24–36
Wilks A (2001) The ShuS protein of Shigella dysenteriae is a heme-sequestering protein that also binds DNA. Arch Biochem Biophys 387:137–142
Wilks A, Schmitt MP (1998) Expression and characterization of a heme oxygenase (Hmu O) from Corynebacterium diphtheriae. Iron acquisition requires oxidative cleavage of the heme macrocycle. J Biol Chem 273:837–841
Wu R, Skaar EP, Zhang R et al (2005) Staphylococcus aureus IsdG and IsdI, heme-degrading enzymes with structural similarity to monooxygenases. J Biol Chem 280:2840–2846
Wyckoff EE, Lopreato GF, Tipton KA, Payne SM (2005) Shigella dysenteriae ShuS promotes utilization of heme as an iron source and protects against heme toxicity. J Bacteriol 187:5658–5664
Zhu W, Hunt DJ, Richardson AR, Stojiljkovic I (2000a) Use of heme compounds as iron sources by pathogenic Neisseriae requires the product of the hemO gene. J Bacteriol 182:439–447
Zhu W, Wilks A, Stojiljkovic I (2000b) Degradation of heme in gram-negative bacteria: the product of the hemO gene of Neisseriae is a heme oxygenase. J␣Bacteriol 182:6783–6790
Acknowledgements
We would like to thank the members of the Skaar lab for critical reading of this manuscript. Work in the Skaar lab is supported by support from the Searle Scholars Program, and United States Public Health Service Grant AI69233 from the National Institute of Allergy and Infectious Diseases. Eric Skaar, Ph.D. holds an Investigators in Pathogenesis of Infectious Disease Award from the Burroughs Welcome Fund. M.L.R. was funded by NIH Training Grant in Mechanisms of Vascular Disease, 5 T32 HL07751. V.J.T. was funded by Ruth L. Kirschstein National Research Service Award AI071487.
Author information
Authors and Affiliations
Corresponding author
Additional information
Since the acceptance of this manuscript, two papers have been published that are directly relevant to this review. The first describes IsdB as a hemoglobin receptor in S.␣aureus: J Bacteriol. 2006 Dec; 188(24): 8421–8429. The second identifies an IsdG-family member in a Gram␣negative bacterium: J Bacteriol. 2006 Sep; 188(18): 6476-6482.
Rights and permissions
About this article
Cite this article
Reniere, M.L., Torres, V.J. & Skaar, E.P. Intracellular metalloporphyrin metabolism in Staphylococcus aureus . Biometals 20, 333–345 (2007). https://doi.org/10.1007/s10534-006-9032-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-006-9032-0