Abstract
The interest in synthetic siderophore mimics includes therapeutic applications (iron chelation therapy), the design of more effective agents to deliver Fe to plants and the development of new chemical tools in order to study iron metabolism and iron assimilation processes in living systems. The design of ligands needs a rational approach for the understanding of the metal ion complexing abilities. The octahedral arrangement of donor atoms is the most favourable geometry, allowing the maximum possible distance between their formal or partial negative charges. Hexadentate chelators, usually of the tris-bidentate type, can accommodate the metal coordination sphere and are well-suited to obtain high pFe values. The first part of this review is dedicated to selected synthetic routes, taking into account (i) the nature of the chelating subunits, connecting groups and spacers, (ii) the water-solubility and hydrophilic/lipophilic balance, (iii) the chirality and (iv) the possibility of grafting probes or vectors. In the second part, we discuss the role of the molecular design on complexing abilities (thermodynamics and kinetics). The bidentate 8-hydroxyquinoline moiety offers an alternative to the usual coordinating hydroxamic acids, catechols and/or α-hydroxycarboxylic acids groups encountered in natural siderophores. The promizing results obtained with the tris-hydroxyquinoline-based ligand O-TRENSOX are summarized. O-TRENSOX exhibits a high and selective affinity for Fe(III) complexation. Its efficiency in delivering Fe to plants, iron mobilization, cell protection, and antiproliferative effects has been evidenced. Other chelators derived from O-TRENSOX (mixed catechol/8-hydroxyquinoline ligands, lipophilic ligands) are also described. Some results question the relevance of partition coefficients to foresee the activity of iron chelators. The development of probes (fluorescent, radioactive, spin labelled) based on the O-TRENSOX backbone is in progress in order to get insights in the complicated iron metabolism processes.
Similar content being viewed by others
References
AM Albrecht-Gary AL Crumbliss (1998) Coordination chemistry of siderophores: thermodynamics and kinetics of iron chelation and release A Sigel H Sigel (Eds) Metal Ions in Biological Systems, Vol. 35: Iron Transport and Storage in Microorganisms, Plants and Animals New-York Maxel Dekker 239–327
Albrecht-Gary AM, Blanc S, Biaso F, Thomas F, Baret P, Gellon G, Pierre JL, Serratrice G. 2003 Iron(III) chelation Tuning of the pH dependence by mixed ligands. Eur J Inorg Chem 2596–2605
M Apostol P Baret G Serratrice J Desbrières JL Putaux MJ Stebé D Expert JL Pierre (2005) ArticleTitleSelf-assembly of an amphiphilic iron(III) chelator: mimicking iron acquisition in marine bacteria Angew Chem Int Ed 44 2580–2582 Occurrence Handle1:CAS:528:DC%2BD2MXktFejtb0%3D Occurrence Handle10.1002/anie.200462841
P Baret C Béguin D Gaude G Gellon C Mourral JL Pierre G Serratrice A Favier (1994) ArticleTitleTripodal ligands possessing six convergent hydroxyl groups. A novel family of iron sequestering agents based on o, o′-dihydroxybiphenyl subunits Tetrahedron 7 2077–2094
P Baret C Béguin H Boukhalfa C Caris JP Laulhère JL Pierre G Serratrice (1995) ArticleTitle O-TRENSOX: A promizing water soluble iron chelator (both Fe(III) and Fe(II)) potentially suitable for plant nutrition and iron chelation therapy J Am Chem Soc 117 9760–9761 Occurrence Handle1:CAS:528:DyaK2MXnvFOks7g%3D Occurrence Handle10.1021/ja00143a021
Baret P, Beaujolais V, Béguin C, Gaude D, Pierre JL, Serratrice G. 1998 Towards new iron(III) chelators: Synthesis and complexing abilitiy of a water-soluble tripodal ligand based on 2, 2′-dihydroxybiphenyl subunits. Eur J Inorg Chem 613–619
Baret P, Béguin C, Gellon G, Pierre JL, Serratrice G, Thomas F, Laulhère JP, Saint-Aman E. 2000 TRENPYPOLS: A new water-soluble iron chelator (both Fe(III) and Fe(II)) involving six-membered coordination ring. Eur J Inorg Chem 1219–1227
RJ Bergeron JS Mc Manis (1991) Synthesis of catecholamide and hydroxamate siderophores G Winkelman (Eds) Handbook of Microbial Iron Chelates CRC Press Boca Raton 271–307
RJ Bergeron MG Xin WR Weimar RE Smith J Wiegand (2001) ArticleTitleSignificance of asymmetric sites in choosing siderophores deferration agents J Med Chem 44 2469–2478 Occurrence Handle11448229 Occurrence Handle1:CAS:528:DC%2BD3MXksVKltLY%3D Occurrence Handle10.1021/jm010019s
F Biaso P Baret JL Pierre G Serratrice (2002) ArticleTitleComparative studies of the iron chelators O-TRENSOX and TRENCAMS: Selectivity towards other biological relevant metal ions and Al3+ J Inorg Biochem 89 123–130 Occurrence Handle11931972 Occurrence Handle1:CAS:528:DC%2BD38XisV2mu7w%3D Occurrence Handle10.1016/S0162-0134(01)00401-9
ME Bluhm SS Kim EA Dertz KN Raymond (2002) ArticleTitleCorynobactin and enterobactin: related siderophore of opposite chirality J Am Chem Soc 124 2436–2437 Occurrence Handle11890782 Occurrence Handle1:CAS:528:DC%2BD38XhtlCmu7Y%3D Occurrence Handle10.1021/ja016651s
Bollinger JE, Mague JT, O’Connor CJ, Banks WA, Roundhill DM. 1995 Lipophilic hexadentate gallium, indium and iron complexes of new phenolate-derivatized cyclohexanetriamines as potential in vivo metal-transfer reagents. J Chem Soc Dalton Trans 1677–1995
ME Cass TM Garrett KN Raymond (1989) ArticleTitleThe salicylate mode of bonding in protonated ferric enterobactin analogs J Am Chem Soc 111 1677–1682 Occurrence Handle1:CAS:528:DyaL1MXhtVKqtb4%3D Occurrence Handle10.1021/ja00187a021
SM Cohen B O’Sullivan KN Raymond (2000) ArticleTitleMixed hydroxypiridinonates ligands as iron chelators Inorg Chem 39 4339–4336 Occurrence Handle11196930 Occurrence Handle1:CAS:528:DC%2BD3cXmtVSqtrg%3D Occurrence Handle10.1021/ic000239g
JB Galey O Destrée J Dumats S Génard P Tachon (2000) ArticleTitleProtection against oxidative damage by iron chelators: effect of lipophilic analogs and prodrugs of N, N′-bis(3, 4, 5-trimethoxybenzyl)ethylenediamine-N, N′-diacetic acid J Med Chem 43 1418–1421 Occurrence Handle10753479 Occurrence Handle1:CAS:528:DC%2BD3cXhslSksbw%3D Occurrence Handle10.1021/jm9911635
TM Garrett ME Cass KN Raymond (1992) ArticleTitleHydrogen bonding in catecholamides J Coord Chem 25 241–253 Occurrence Handle1:CAS:528:DyaK38XktlOhtrY%3D Occurrence Handle10.1080/00958979209409197
Y Hara M Akiyama (2001) ArticleTitleAn iron reservoir model based on ferrichrome: Iron(III)-binding and metal(III)-exchange properties of tripodal monotopic and ditopic hydroxamate ligands with an l-alanyl-l-alanyl-N-hydroxy-β-alanyl sequence J Am Chem Soc 123 7247–7256 Occurrence Handle11472152 Occurrence Handle1:CAS:528:DC%2BD3MXkvFGju7k%3D Occurrence Handle10.1021/ja003251g
WR Harris KN Raymond FL Weitl (1981) ArticleTitleFerric ion sequestering agents 6. The spectrophotometric and potentiometric evaluation of sulfonated tricatecholate ligands J Am Chem Soc 103 2667–2675 Occurrence Handle1:CAS:528:DyaL3MXktFelsb8%3D Occurrence Handle10.1021/ja00400a030
BP Hay DA Dixon R Vargas J Garza KN Raymond (2001) ArticleTitleStructural criteria for the rational design of selective ligands. 3: Quantitative structure-stability relationship for iron(III) complexation by tris-catecholamide siderophores Inorg Chem 40 3922–3995 Occurrence Handle11466050 Occurrence Handle1:CAS:528:DC%2BD3MXks1ymurg%3D Occurrence Handle10.1021/ic001380s
C Henry N Rabka D Imbert F Thomas P Baret G Serratrice D Gaude JL Pierre R Ward RR Crichton G Lescoat (2001) ArticleTitleNew 8-hydroxyquinoline and catecholate iron chelators: influence of their partition coefficient on their biological activity Biochem Pharmacol 62 1355–1362 Occurrence Handle11709195 Occurrence Handle1:CAS:528:DC%2BD3MXot1Ols7c%3D Occurrence Handle10.1016/S0006-2952(01)00779-1
RC Hider JB Porter S Singh (1994) The design of therapeutically useful iron chelators RJ Bergeron GM Brittenham (Eds) The Development of Iron Chelators for Clinical Use CRC Press Boca Raton 353–371
D Imbert F Thomas P Baret G Serratrice D Gaude JL Pierre JP Laulhère (2000) ArticleTitleSynthesis and iron(III) complexing ability of CacCAM, a new analogue of enterobactin possessing a free carboxylic anchor arm. Comparative studies with TRENCAM New J Chem 24 281–288 Occurrence Handle1:CAS:528:DC%2BD3cXislOkt7Y%3D Occurrence Handle10.1039/b000229l
D Imbert P Baret D Gaude I Gautier-Luneau G Gellon F Thomas G Serratrice JL Pierre (2002) ArticleTitleHydrophilic and lipophilic iron chelators with the same complexing abilities Chem Eur J 8 1091–1100 Occurrence Handle1:CAS:528:DC%2BD38Xit1GmsLk%3D Occurrence Handle10.1002/1521-3765(20020301)8:5<1091::AID-CHEM1091>3.0.CO;2-Y
ZD Liu RC Hider (2002) ArticleTitleDesign of chelators with therapeutic application Coord Chem Rev 232 151–171 Occurrence Handle1:CAS:528:DC%2BD38XntF2kur4%3D Occurrence Handle10.1016/S0010-8545(02)00050-4
M Meyer JR Telford SM Cohen DJ White J Xu KN Raymond (1997) ArticleTitleHigh yield synthesis of the enterobactin trilactone and evaluation of derivative siderophores analogues J Am Chem Soc 119 10093–10103 Occurrence Handle1:CAS:528:DyaK2sXms1Cku7s%3D Occurrence Handle10.1021/ja970718n
MM Meijler A Arad-Yellin ZI Cabantchik A Shanzer (2002) ArticleTitleSynthesis and evaluation of iron chelators with masked hydrophilic moieties J Am Chem Soc 124 12666–12667 Occurrence Handle12392406 Occurrence Handle1:CAS:528:DC%2BD38Xns1SlsL4%3D Occurrence Handle10.1021/ja027013s
MJ Miller F Malouin (1993) ArticleTitleMicrobial iron chelators as drug delivery agents: the rational design and synthesis of siderophore-drug conjugates Acc Chem Res 26 241–249 Occurrence Handle1:CAS:528:DyaK3sXitF2msbg%3D Occurrence Handle10.1021/ar00029a003
CY Ng SJ Rodgers KN Raymond (1989) ArticleTitleFerric ion sequestering agents, 21. Synthesis, spectroscopic and potentiometric evaluation of trihydroxamate analogs of ferrichrome Inorg Chem 28 2062–2066 Occurrence Handle1:CAS:528:DyaL1MXitF2qtb8%3D Occurrence Handle10.1021/ic00310a011
JL Pierre P Baret G Serratrice (2003) ArticleTitleHydroxyquinolines as iron chelators Current Med Chem 10 1077–1084 Occurrence Handle1:CAS:528:DC%2BD3sXjs1yjtbs%3D Occurrence Handle10.2174/0929867033457584
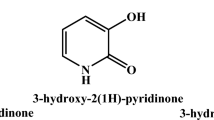
BL Rai H Khodr RC Hider (1999) ArticleTitleSynthesis, physico-chemical and iron(III)-chelating properties of novel hexadentate 3-hydroxy-2-(1H) pyridinone ligands Tetrahedron 55 1129–1142 Occurrence Handle1:CAS:528:DyaK1MXhtFyntLc%3D Occurrence Handle10.1016/S0040-4020(98)01091-6
DR Richardson P Ponka (1998) ArticleTitlePyridoxal isonicotinoyl hydrazone and its analogs: potential orally effective iron-chelating agents for the treatment of iron overload disease J Lab Clin Med 131 306–315 Occurrence Handle9579383 Occurrence Handle1:CAS:528:DyaK1cXjtFyhu7c%3D Occurrence Handle10.1016/S0022-2143(98)90180-9
JM Rosenberg YM Lin Y Lu MJ Miller (2000) ArticleTitleStudies and syntheses of siderophores, microbial iron chelators, and analogs as potential drug delivery agents Current Med Chem 7 159–197
A Shanzer J Libman (1991) Biomimetic siderophores G Winkelman (Eds) Handbook of Microbial Iron Chelates CRC Press Boca Raton 309–338
A Shanzer J Libman (1998) Biomimetic siderophores: From structural probes to diagnostic tools A Sigel H Sigel (Eds) Metal Ions in Biological Systems, Vol. 35: Iron Transport and Storage in Microorganisms, Plants and Animals Maxel Dekker New-York 329–354
G Serratrice H Boukhalfa C Béguin P Baret C Caris JL Pierre (1997) ArticleTitle O-TRENSOX, a new tripodal iron chelator based on 8-hydroxyquinoline subunits: Thermodynamic and kinetic studies Inorg Chem 36 3898–3910 Occurrence Handle1:CAS:528:DyaK2sXlsVOgsbo%3D Occurrence Handle10.1021/ic9608096
G Serratrice P Baret H Boukhalfa I Gautier-Luneau JL Pierre (1999) ArticleTitleStructural characterization of a tris-salicylate coordination for iron(III) with the tripodal ligand O-TRENSOX Inorg Chem 38 840–841 Occurrence Handle11670851 Occurrence Handle1:CAS:528:DyaK1MXhtFagtrg%3D Occurrence Handle10.1021/ic980402w
G Serratrice SB Galey E Saint-Aman J Dumats (2001) ArticleTitleIron(III) complexation by new aminocarboxylate chelators-thermodynamic and kinetic studies Eur J Inorg Chem 2 471–479 Occurrence Handle10.1002/1099-0682(200102)2001:2<471::AID-EJIC471>3.0.CO;2-A
G Serratrice F Biaso F. Thomas C. Béguin (2004) ArticleTitleMechanism of stepwise dissociation of Fe(III) complexes with tripodal ligands as siderophores models Eur J Inorg Chem 7 1552–1565 Occurrence Handle10.1002/ejic.200300396 Occurrence Handle1:CAS:528:DC%2BD2cXjt1emu74%3D
A Shanzer J Libman S Lifson (1992) ArticleTitleMultiple weak forces in ion-binding molecules Pure & Appl Chem 64 1421–1435 Occurrence Handle1:CAS:528:DyaK3sXjtlai
M Streater PD Taylor RC Hider J Porter (1990) ArticleTitleNovel 3-hydroxy-2(1H)-pyridinones. Synthesis, iron(III)-chelating properties and biological activity J Med Chem 33 1749–1755 Occurrence Handle2342069 Occurrence Handle1:CAS:528:DyaK3cXit12lur0%3D Occurrence Handle10.1021/jm00168a033
Telford JR, Raymond KN. 1996 Siderophores, In: Comprehensive Supramolecular Chemistry 1, 245–266
Y Sun RJ Motekaitis AE Martelle (1998) ArticleTitleA novel iron sequestering agent: synthesis and iron-chelating properties of 1, 1, 1-tris(3-hydroxo-2-oxo-1, 2-dihydro-1-pyridylpropoxy methyl)-ethane Inorg Chim Acta 281 60–63 Occurrence Handle1:CAS:528:DyaK1cXmt1KltL4%3D Occurrence Handle10.1016/S0020-1693(98)00143-1
F Thomas C Béguin JL Pierre G Serratrice (1999) ArticleTitleThermodynamic and kinetic studies of the sulfonated derivative of the iron chelator TRENCAM, an analog of enterobactin Inorg Chim Acta 291 148–157 Occurrence Handle1:CAS:528:DyaK1MXmtVSqsr8%3D Occurrence Handle10.1016/S0020-1693(99)00130-9
F Thomas P Baret D Imbert J Pierre G Serratrice (1999) ArticleTitlePartition coefficients (Free ligands and their iron(III) complexes) and lipophilic behavior of new abiotic chelators. Correlation to biological activity Bioorg Med Chem Lett 9 3035–3040 Occurrence Handle10571171 Occurrence Handle1:CAS:528:DyaK1MXntFKlu74%3D Occurrence Handle10.1016/S0960-894X(99)00527-2
GS Tilbrook RC Hider (1998) Iron chelators for clinical use A Sigel H Sigel (Eds) Metal Ions in Biological Systems, Vol 35: Iron Transport and Storage in Microorganisms, Plants and Animals Maxel Dekker New-York 691–730
Y Tor J Libman A Shanzer S Lifson (1987) ArticleTitleBiomimetic ferric iron carriers. A chiral analog of enterobactin J Am Chem Soc 109 6517–6518 Occurrence Handle1:CAS:528:DyaL2sXmtFyjsb0%3D Occurrence Handle10.1021/ja00255a050
FL Weitl KN Raymond (1981) ArticleTitleLipophilic enterobactin analogs. Terminally N-alkylated spermine/spermidine catecholcarboxamides J Org Chem 46 5234–5237 Occurrence Handle1:CAS:528:DyaL38XhvFOksg%3D%3D Occurrence Handle10.1021/jo00338a042
F Yunta S Garcia-Marco JJ Lucena M Gomez-Gallego R Alcazar MA Sierra (2003) ArticleTitleChelating agents related to ethylenediamine-tris(2-hydroxyphenyl)acetic acid (EDDHA): synthesis, characterization, and equilibrium studies of the free ligands and their Mg2+, Ca2+, Cu2+, and Fe3+ chelates Inorg Chem 42 5412–5421 Occurrence Handle12924915 Occurrence Handle1:CAS:528:DC%2BD3sXlvVGlu74%3D Occurrence Handle10.1021/ic034333j
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
d’Hardemare, A.d.M., Torelli, S., Serratrice, G. et al. Design of Iron chelators: Syntheses and iron (III) complexing abilities of tripodal tris-bidentate ligands. Biometals 19, 349–366 (2006). https://doi.org/10.1007/s10534-005-2997-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10534-005-2997-2