Abstract
Soil drying and wetting cycles can produce pulses of nitric oxide (NO) and nitrous oxide (N2O) emissions with substantial effects on both regional air quality and Earth’s climate. While pulsed production of N emissions is ubiquitous across ecosystems, the processes governing pulse magnitude and timing remain unclear. We studied the processes producing pulsed NO and N2O emissions at two contrasting drylands, desert and chaparral, where despite the hot and dry conditions known to limit biological processes, some of the highest NO and N2O flux rates have been measured. We measured N2O and NO emissions every 30 min for 24 h after wetting soils with isotopically-enriched nitrate and ammonium solutions to determine production pathways and their timing. Nitrate was reduced to N2O within 15 min of wetting, with emissions exceeding 1000 ng N–N2O m−2 s−1 and returning to background levels within four hours, but the pulse magnitude did not increase in proportion to the amount of ammonium or nitrate added. In contrast to N2O, NO was emitted over 24 h and increased in proportion to ammonium addition, exceeding 600 ng N–NO m−2 s−1 in desert and chaparral soils. Isotope tracers suggest that both ammonia oxidation and nitrate reduction produced NO. Taken together, our measurements demonstrate that nitrate can be reduced within minutes of wetting summer-dry desert soils to produce large N2O emission pulses and that multiple processes contribute to long-lasting NO emissions. These mechanisms represent substantial pathways of ecosystem N loss that also contribute to regional air quality and global climate dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil drying–wetting cycles are widespread and can stimulate large emissions of both nitrous oxide (N2O) and nitric oxide (NO) (Scholes et al. 1997; Homyak et al. 2016; Eberwein et al. 2020) with profound implications for Earth’s climate, regional air quality, and ecosystem N retention. This is because N2O is a potent greenhouse gas (Ciais et al. 2013), NO is a precursor to tropospheric ozone (Crutzen 1979), and both NO and N2O represent important pathways for ecosystem N loss (Peterjohn and Schlesinger 1990). While the production of NO and N2O is governed by both biological and chemical processes upon wetting dry soil, the magnitude of the emissions vary as a function of aridity (Wang et al. 2014; Liu et al. 2017; von Sperber et al. 2017), with some drylands recording among the highest NO and N2O emission pulses globally (Eberwein et al. 2020). However, how these emissions vary across ecosystems experiencing drying–wetting cycles and the biogeochemical processes producing them are still not well characterized. Identifying the underlying processes producing pulsed NO and N2O emissions is necessary to predict how ecosystem N cycling may respond to global change factors including high rates of atmospheric N deposition (Fenn et al. 2006), rising temperatures, and changing precipitation regimes (Dai 2013).
Multiple biological and abiotic processes regulate NO and N2O emissions after dry soils are wetted. Biological processes include nitrification, the aerobic oxidation of ammonia (NH3) to nitrate (NO3−) with NO and nitrite (NO2−) as intermediates (Caranto and Lancaster 2017; Prosser et al. 2019), and denitrification, the sequential anaerobic reduction of NO3− to N2 gas with NO2−, NO, and N2O as obligate intermediates (Knowles 1982); both of these processes can release NO and N2O as byproducts. In low oxygen environments, some nitrifiers use NO2− as the electron acceptor during the oxidation of NH3 and produce NO and N2O via nitrifier denitrification (Prosser et al. 2019). Chemodenitrification—an abiotic non-enzymatic process—can also produce NO and N2O through the chemical reduction of NO2− and hydroxylamine (NH2OH) (Venterea and Rolston 2000; Zhu-Barker et al. 2015; Heil et al. 2016), which can accumulate in dry soils (Homyak et al. 2016). Because both biological and abiotic processes can occur simultaneously, it has been challenging to determine the contribution of individual processes to pulsed N emissions.
To advance understanding of the processes producing NO and N2O, the “hole-in-the-pipe” conceptual framework relates the factors that control N emissions to the processes that control N transformation rates (Firestone and Davidson 1989). Under this framework, N transformations are represented by changes in the diameter of the pipe—the diameter varies in proportion to process rates—whereas the factors controlling how much NO or N2O leak out of the pipe are represented by the holes (e.g., edaphic or environmental factors such as pH). In this sense, wetting soils could stimulate NO and N2O emissions by promoting nitrification, (Placella and Firestone 2013; Homyak and Sickman 2014), denitrification (Parker and Schimel 2011; Soper et al. 2016), or abiotic reactions (McCalley and Sparks 2009; Zhu-Barker et al. 2015; Homyak et al. 2017), thereby increasing the diameter of the pipe. However, the magnitude and timing of pulsed N emissions may vary as a function of environmental and edaphic factors that mediate which gaseous N intermediates are released to the atmosphere (i.e., the holes in the pipe). Understanding how process rates interact with the factors that control how much NO and N2O are emitted can help determine how N emissions may vary under future global change scenarios.
Two major challenges have limited progress identifying controls over soil NO and N2O emission pulses: (i) multiple biological and abiotic processes occur simultaneously making them difficult to separate, and (ii) traditional static chamber headspace experiments offer low temporal resolution, limiting understanding of the timing and magnitude of N trace gas emissions. To this end, isotope tracers are powerful tools that can help determine which N transformations produce NO and/or N2O (Van Groenigen et al. 2015). Moreover, isotope tracers can be coupled with laser-based isotope analyzers and automated soil chambers to detect the incorporation of 15N tracers into N2O in-situ and at high resolution (e.g., one measurement per second). While similar instruments do not yet exist for NO, the incorporation of 15N tracers into NO can be detected using passive samplers (Homyak et al. 2016). By pairing high temporal resolution measurements of N emissions with stable isotopes, we assess the importance of increasing N availability (here used as a proxy for increasing the diameter of the pipe) relative to the factors that control how much NO and N2O is released (i.e. the holes in the pipe). Specifically, we ask: (1) what processes are contributing to pulsed NO and N2O emissions after wetting dry soils, and (2) how does N availability (both the amount and chemical form) affect the magnitude of pulsed emissions?
To answer these questions, we monitored N emissions at two dryland sites (desert and chaparral) in Southern California characterized by pronounced and frequent transitions from dry-to-wet soils. We chose two sites with contrasting environmental conditions (Table 1) to understand whether meteorological and edaphic factors would overrule the effects of increasing N supply and the form of nitrogen added, nitrate (NO3−) or ammonium (NH4+). We hypothesized that N trace gas emissions are limited by soil N availability, resulting in pulsed NO and N2O emissions proportional to the amount of added N. To infer which processes contributed to NO and N2O emissions, we added 15N labeled NO3− or NH4+ and used an automated chamber system connected to a NO and an isotope N2O analyzer. We also measured NH3 emissions using passive samplers as a relative index of the amount of NH3 in soil pore space that may be available to nitrifiers. We predicted that if pulsed N emissions were from nitrification, then added 15N–NH4+ would be captured as NO and/or N2O; if they were from denitrification, then added 15N–NO3− would be captured as NO and/or N2O; and if they were from the rapid transformation of accumulated nitrification intermediates (e.g., NO2−), then no 15N label would be incorporated in N emissions.
Methods
Sites description
We studied two drylands in Southern California in August 2018 (the end of the summer dry season) with contrasting soils and vegetation. Our chaparral site was located in the Box Springs Reserve (33° 58′ 16.4″ N, 117° 17′ 53.4″ W), a transitional zone between coastal sage scrub and chaparral dominated by chamise (Adenostoma fasciculatum). Our desert site was located in the Boyd Deep Canyon Desert Research Center (33° 38′ 54.7″ N, 116° 22′ 39.4″ W), and was dominated by creosote (Larrea tridentata). Both sites are part of the University of California Natural Reserve System. Since 1980, the chaparral site has received an average of 28 cm of rain per year with an average maximum August daily temperature of 35 °C. During this same time, the desert site received an average of 11.4 cm of rain per year with an average maximum August daily temperature of 39.3 °C. The chaparral soils are sandy loams classified as thermic Typic Haploxeralfs within the Fallbrook series. The desert soils are stony sands classified as hyperthermic Typic Torriorthents within the Carrizo series. Both sites received no rain in the month before our experiments.
The soils at the two sites differed in several ways (Table 1). Soil NO2− was over seven times greater in the desert (0.58 ± 0.64 µg N g−1) than in the chaparral (0.08 ± 0.03 µg N g−1, p < 0.05), while extractable NO3− and NH4+ did not differ between sites (Table 1). Total C and N concentrations were both higher in the chaparral (2.03 ± 0.35% C, 0.15 ± 0.02% N) than in the desert (0.92 ± 0.50% C, 0.08 ± 0.03% N). Desert soils were more alkaline (8.4 ± 0.19) than the chaparral (5.8 ± 0.50, p < 0.05). In the hour before starting our experiment, relative humidity was higher in the chaparral (80.7 ± 15.0%) relative to the desert (10.7 ± 1.04%, p < 0.001), while soil temperature was higher in the desert (30.0 ± 0.68 °C) than in the chaparral (16.5 ± 2.15 °C, p < 0.001).
Experimental design
We measured N trace gas emissions from underneath eight chamise shrubs in the chaparral and eight creosote shrubs in the desert. Interspace soils were not sampled as dryland shrubs are considered to be “islands of fertility” where soil nutrients are concentrated (Schlesinger et al. 1990). All shrubs were located within a 10-m radius and were separated from one another by at least one meter. Under each of the eight shrub canopies, we installed two pairs of PVC collars (4 collars, each 20 cm diameter × 10 cm height; inserted 5 cm into the ground) at least 48 h prior to the start of our measurements; the collar pairs were separated from each other by at least 50 cm to avoid cross contamination of isotope tracer and within 50 cm from the base of the shrubs. One pair of collars was wetted with NO3− solution, while the other was wetted with NH4+ solution. Within each pair, one collar was used to measure N emissions, while the other was used to measure soil temperature, moisture, and inorganic N to minimize disturbances to the collars from which we measured emissions.
We wetted soils inside the collars with 500 mL of deionized water; this amount corresponds to about seven mm of rainfall, which is within the range of historically occurring rain events (Boyd Deep Canyon Desert Research Station, https://doi.org/10.21973/N3V66D). During wetting, we added eight levels of N spike corresponding to 0, 2, 4, 6, 8, 10, 12, or 15 kg-N ha−1 as either NO3− or NH4+, covering a range of annual N deposition in Southern California drylands (Eberwein et al. 2020, Fenn et al. 2006). The nitrogen added was isotopically enriched to 2 atom percent 15N. The labeled NO3− was added to two of the collars underneath each shrub starting at approximately 9 am. Soil NO and N2O emissions were measured from one collar underneath each shrub every 30 min beginning 15 min prior to wetting. After 24 h, this process was repeated with the NH4+ label using the remaining collars underneath each shrub.
A separate group of four shrubs was used to measure the emission of NH3 as well as the isotopic composition of NO. Emissions of NH3 were used as an index of substrate availability to nitrifiers. These measurements were made using passive samplers (Ogawa pads; Ogawa USA, Pompano Beach, FL) that required soil chambers to be permanently closed, prohibiting integration with our automated chambers. The passive sampling pads are chemically pretreated so that they would collect either NOx, NO2, or NH3 and have been demonstrated to work well under warm and humid conditions expected inside our soil chambers (Coughlin et al. 2017). We did not detect NO2 on the NO2 pads, indicating any N accumulation on the NOx pads was mostly NO. Two collars underneath each of the shrubs were wetted with 500 mL of either NO3− or NH4+ solution (2 atom percent 15N) at a concentration corresponding to 15 kg-N ha−1. The remaining two collars underneath each of the four shrubs were wetted with deionized water only. Chamber lids were installed immediately after wetting and pads were switched out at the following time intervals: 0 to 15 min, 15 min to 12 h, and 12 to 24 h post-wetting to capture NO and NH3 during periods when we expected N emissions to be high.
NO and N2O emissions
We used an automated chamber system to simultaneously measure NO and N2O emissions from one of the collars under each of the eight shrubs sequentially over a 24-h period post-wetting. Collars were equipped with automated chambers (8100-104/C, LI-COR Biosciences, Lincoln, NE) connected to a multiplexer (LI-8150, LI-COR Biosciences) to sequentially measure emissions from each of the eight collars. We measured gas concentrations for two minutes, during which time gas from the chamber was recirculated through a sample loop connecting the multiplexer, an infrared gas analyzer (IRGA; LI-8100, LI-COR Biosciences), an isotope N2O analyzer (Model 914-0027, Los Gatos Research, Inc., Mountain View, CA), and a NO analyzer (Model 410 and Model 401, 2B Technologies, Boulder CO). The IRGA, N2O analyzer, and NO analyzer all sampled air from the recirculating sample loop, and each instrument, except for the NO analyzer, returned air back into the sample loop. Since the NO analyzer consumed NO, this air was vented to the atmosphere at a rate of 0.75 L min−1. While this open system dilutes the concentration of trace gases emitted from the soil with atmospheric air, flux rates are not appreciably affected after accounting for our chamber volume (~ 6 L) and the short incubation period (Davidson et al. 1991). All instruments were housed inside an air-conditioned box made of five cm thick housing insulation (5 × 2 × 2 m). To prevent condensation in the lines, the sample loop included a water trap to remove moisture by cooling the hoses with ice water. Soil temperature (Model 8150-203, LI-COR Biosciences) and moisture sensors (Model 8150-205, LI-COR Biosciences) were installed under each shrub and were connected to the IRGA, which also measured relative humidity.
Fluxes of NO and N2O were calculated as the linear change in trace gas concentrations inside the chamber headspace over the last 90 s of the two-minute incubation (script available on https://github.com/handr003/TraceGasArray). This timeframe was chosen to allow for even mixing of chamber air throughout the sample loop. The N2O analyzer recorded concentrations once every second and the NO analyzer recorded every ten seconds. If the linear correlation between time and trace gas concentration was not statistically significant (p > 0.1), the net flux was reported as zero. The change in NO concentration over time was highly linear over the 90 s window for all measurements (R2 = 0.96). The change in N2O concentration over time was close to linear for all measurements (R2 = 0.56) and was highly linear when N2O fluxes were greater than 10 ng N–N2O m−2 s−1 (R2 = 0.97).
Flux values were corrected for the volume in the sample loop, soil temperature, and chamber volume. The isotopic N2O analyzer also recorded [δ15N]N2O, which requires five minutes of averaging time to report δ15N values within 1-sigma precision. Given the short incubation period of our measurements (2 min) and the fact that our measurements were diluted with ambient air, we do not attempt to calculate absolute [δ15N]N2O values. Rather, we report *[δ15N]N2O as an index of when 15N tracer was incorporated into N2O after wetting dry soils. *[δ15N]N2O was calculated as the average δ15N value during the final 10 s of each incubation—across all measurements the standard deviation of [δ15N]N2O during this 10 s interval averaged 4.95 ‰. We also refrain from reporting isotopomer values for these same reasons—two-minute chamber closures were not sufficient to ensure isotopic accuracy and precision. The isotope N2O analyzer was referenced against a commercially available standard (Airgas, 5000 ppm N2O, δ15N = − 0.3 ‰) and a cylinder of medical grade air analyzed for N2O and isotopic composition at the UC Davis Stable Isotope Facility (0.44 ± 0.02 ppm N2O; δ15N = 5.76 ± 0.15 ‰).
NO isotopes and soil NH3 emissions
We used the bacterial denitrifier method to measure the [δ15N]NO and [δ18O]NO of NO captured on the NOx pads (Coplen et al., 2012). Briefly, the Ogawa pads were extracted in 8 mL of deionized water and shaken overnight to extract NO as NO2−; no NO3− was detected in the filtered extracts. The NO2− was then converted to N2O using Pseudomonas aureofaciens (Sigman et al. 2001). δ15 N and δ18O values were measured using a Thermo Delta V isotope ratio mass spectrometer (Thermo Fisher Scientific, Woltham, MA) at the Facility for Isotope Ratio Mass Spectrometry (FIRMS; https://ccb.ucr.edu/facilities/firms) at the University of California, Riverside. Due to isotopic fractionation associated with NO collection with passive samplers, isotopic fractionation associated with the denitrifier method, exchange of oxygen atoms between NO2− and water (Casciotti et al. 2007; Dahal and Hastings, 2016; Yu and Elliott 2017), and potential interactions between volatile organic compounds (VOC) with NO (Walters and Michalski 2016) it is unlikely we measured the actual [δ15N]NO and [δ18O]NO emitted from soil. However, these fractionation and oxygen exchange effects are generally uniform across samples (Dahal and Hastings, 2016) and even if NO and VOCs interacted within our chambers, the passive samplers can still inform when 15N tracers added to soils are detected as NO (Homyak et al. 2016), altogether helping to preserve a [δ15N]NO and [δ18O]NO signal from the Ogawa pads, hereafter referred to as *[δ15N]NO and *[δ18O]NO. To help preserve the [δ18O]NO signal from the Ogawa pads, we used the same water source to prepare all isotope tracers and analyzed all samples in a single batch. Furthermore, our samples did not have NO3−—the NO was extracted as NO2−—reducing bias in the final isotope measurement (Casciotti et al. 2007).
We used Ogawa pads to measure NH3 emissions as an index of NH3 availability in soil pore space that may be available to nitrifiers. The NH3 pads were extracted in 8 mL of deionized water and shaken overnight to extract NH3 as NH4+. Extracted NH4+ was quantified using a colorimetric assay (SEAL methods Environmental Protection Agency (EPA)-126-A) using a SEAL AQ-2 discrete analyzer (SEAL analytical, Mequon, WI).
Soil chemical properties
We measured soil extractable NH4+ and NO3− prior to wetting, two hours after wetting, and 24 h after wetting. NO3− and NH4+ were measured by extracting soils (5 g) in 2 M KCl (30 mL). Soil solutions were shaken for one hour, filtered (Whatman 42 filter paper; 2.5 µm pore size), and frozen until analysis. We also measured NO2− prior to wetting; NO2− was extracted in deionized water to minimize its loss via gaseous N products (Homyak et al. 2015). We used colorimetric assays to measure soil extractable NH4+ (SEAL method EPA-126-A), NO3− (SEAL method EPA-129-A), and NO2− (SEAL method EPA-137-A). Additionally, we measured total C, total N, and pH in dry soils (0–10 cm depth) collected from underneath each shrub prior to adding water or N. Soil total C and total N was measured in an elemental analyzer (Flash EA1112; Thermo Scientific, Woltham, MA) at the Environmental Sciences Research Laboratory at the University of California, Riverside (https://envisci.ucr.edu/research/environmental-sciences-research-laboratory-esrl). Soil pH was measured in a 1:1 soil to water ratio with a pH meter (Orion VersaStar Pro; Thermo Scientific, Woltham, MA).
Statistical analyses
All statistics were conducted in R version 3.6.1 (R core development team, 2019). We used linear regression to evaluate the relationship between the amount of added N and soil NO emissions, N2O emissions, and peak *[δ15N]N2O. This was accomplished by first calculating the cumulative NO or N2O emissions measured at each shrub using the “trapz” function. Peak *[δ15N]N2O was calculated as the highest *[δ15N]N2O value recorded from underneath each shrub. We then used the “lm” function to determine the linear relationship between the amount of added N and cumulative NO or N2O emissions and peak *[δ15N]N2O. We report the R2 of each linear regression where p < 0.10 to avoid type II error associated with high spatial variation in field experiments. However, we consider linear regressions with p > 0.05 as “weak” and include alternative explanations for these relationships. A block in the sample loop prevented us from measuring fluxes from two of the collars in the chaparral (2 and 10 kg-N NO3− ha−1) and these data were omitted from our analyses.
We used mixed effects models to determine when 15N tracers were detected in NO collected using passive samplers. The models included *[δ15N]NO as the response variable, collection time as the predictor variable, and a random effect to account for measuring the same collar repeatedly. Models were run using the “nlme” package in R. We used the anova.lme function to determine if time was a significant model term and Tukey corrected multiple comparisons to determine which times differed compared to ambient *[δ15N]NO. We used the same approach to determine if *[δ15N]NO and *[δ18O]NO changed in collars that were amended with water only.
Results
Soil N2O emissions
In the desert, peak N2O emissions averaged 529 ± 469 ng N–N2O m−2 s−1 after wetting soils with NO3− and NH4+ amended solutions, and returned to prewetting levels within four hours (Fig. 1a,c). However, desert N2O emissions did not increase in proportion to adding more NO3− (p = 0.12) or NH4+ (p = 0.89, Table 2). In contrast to the desert, peak chaparral N2O emissions averaged only 38.0 ± 72.0 ng N–N2O m−2 s−1 after wetting with NO3− and NH4+amended solutions (Fig. 1b,d). As observed in the desert, N2O emissions did not increase in proportion to adding NO3− (p = 0.25) or NH4+ (p = 0.10, Table 2).
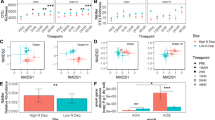
Rapid reduction of NO3− produced pulsed N2O emissions at both sites. In response to 15 kg ha−1 equivalent NO3− addition, *[δ15N]N2O reached 1953 ‰ in the desert and 124 ‰ in the chaparral (Fig. 2a,b), whereas the *[δ15N]N2O of from soils amended with water only did not surpass 32.4 ‰ at either site. Peak *[δ15N]N2O increased in proportion to NO3− addition in the desert (R2 = 0.62, slope = 107 ‰ (kg N ha−1)−1, p = 0.013) and to a smaller degree in the chaparral (R2 = 0.31, slope = 6.2 ‰ (kg N ha−1)−1, p = 0.089, Table S1). *[δ15N]N2O remained under 75 ‰ in NH4+-amended soils at both sites (Fig. 2c,d). Peak *[δ15N]N2O was positively correlated to NH4+ addition in the desert only (R2 = 0.50, slope = 2.85 ‰ (kg N ha−1)−1, p = 0.03), but the slope of this relationship was over 37 times smaller compared to NO3− addition (Table S1).
Isotopic composition (*[δ-15N]N2O) of N2O emitted over 24 h from the desert (a, c) and chaparral (b, d) following wetting of dry soils with nitrate (NO3−; a, b) or ammonium (NH4+; c, d) solutions. Each black dot represents the average isotopic composition of N2O measured over the last 30 s from each chamber
Soil NO and NH3 emissions
Prior to wetting, NO emissions were greater in the chaparral (14.7 ± 4.31 ng N–NO m−2 s−1) than in the desert (0.39 ± 9.27 ng N–NO m−2 s−1) (Table 1). In the desert, NO emissions steadily increased for 10 h post-wetting, and remained elevated for the remainder of the experiment (Fig. 3a); peak NO emissions averaged 221 ± 269 ng N–NO m−2 s−1 in NO3−-amended soils (Fig. 3a) and 254 ± 200 ng N–NO m−2 s−1 in NH4+-amended soils (Fig. 3c). In contrast to the desert, chaparral NO emissions reached their peak within only 5 h of wetting and decreased at faster rates; NO emissions averaged 114 ± 204 ng N–NO m−2 s−1 in NO3− amended soils (Fig. 3b) and 202 ± 154 ng N–NO m−2 s−1 in NH4+ amended soils (Fig. 3d).
In contrast to N2O, isotopically labeled NH4+ and NO3− were both incorporated into the NO emitted at both sites. The 15N labeled NO3− was rapidly converted to NO in the chaparral (F3,9 = 93.8, p < 0.0001), enriching *[δ15N]NO from − 13.2 ± 1.82 ‰ to 388 ± 27.8 ‰ within 15 min of tracer addition (p < 0.0001, Fig. 4b). In the desert, the 15N–NO3− label was detected in NO (F3,9 = 1.7, p = 0.001) but not at 15 min (*[δ15N]NO = 23.1 ± 7.33 ‰, p = 1.00); it was detected between 0.25 and 12 h, when *[δ15N]NO reached 745 ± 202 ‰ (p = 0.003; Fig. 4a). The 15N–NH4+ label took between 0.25 and 12 h to become incorporated into NO at both sites; *[δ-15N]NO reached 949 ± 152 ‰ in the desert (F3,9 = 12.1, p = 0.002; Fig. 4c) and 754 ± 132 ‰ in the chaparral (F3,9 = 60.4, p < 0.001; Fig. 4d).
Isotopic signature (*[δ-15N]NO) of NO emitted from the desert (a, c) and chaparral (b, d) over 24 h after wetting dry soils with nitrate (NO3−; a, b) or ammonium (NH4+; c, d) solutions. Lines represent the mean [*δ-15N]NO (n = 4) from each treatment within each site. Dots represent individual measurements using passive Ogawa samplers. Asterisks indicate if the mean for a given time differed from the control (*p < 0.1, **p < 0.05, ***p < 0.01)
The natural abundance δ15N– and δ18O–NO values emitted from soils amended with only deionized water decreased over the course of the experimental incubation (Fig. 5). The *[δ18O]NO decreased from approximately 10 ‰ prior to wetting to − 15 ‰ 24 h after wetting in both the chaparral (F3,21 = 7.35, p = 0.002) and the desert (F3,21 = 11.5, p = < 0.001). Similarly, *[δ15N]NO decreased from approximately − 10 ‰ to − 40 ‰ over the course of the incubation in both the chaparral (F3,21 = 5.29, p = 0.007) and the desert (F3,21 = 5.15, p = 0.01).
Dual isotope plot of NO (*[δ-15N]NO and *[δ-18O]NO) produced after wetting dry soils from the desert (a) and chaparral (b) with 500 mL water. Colors correspond to timing of the isotopic signature of NO collected from ambient air and from soils after wetting. Each dot represents the isotopic composition of NO measured at each of the shrubs (n = 8). Isotopic NO composition is presented from shrubs receiving water-only additions
Soil NO emissions increased in proportion to incremental NH4+ additions in the chaparral (R2 = 0.58, p = 0.03, Fig. 6d), whereas in the desert, the relationship was positive but weak (R2 = 0.45, p = 0.07, Fig. 6c). Adding NO3− may have increased NO emissions in the chaparral, but the relationship was weak (p = 0.09, Table 2); adding NO3− did not increase NO emissions in the desert (p = 0.28, Table 2).
Cumulative soil NO emissions (µg N–NO m−2) from the desert (a, c) and chaparral (b, d) over 24 h after wetting dry soils with nitrate (NO3−; a, b) or ammonium (NH4+; c, d) solutions. Lines show the linear regression between added N and cumulative NO emissions. Shaded gray areas represent the 95% confidence interval for statistically significant linear regressions (p < 0.1)
Soil NH3 emissions increased immediately after wetting both sites but remained higher in the desert relative to the chaparral (Fig S1). In the desert, NH3 emissions averaged 27.3 ± 24.6 µg N–NH3 m−2 h−1 between 0.25 and 12 h in NO3− amended soils; the NO3− treatment did not increase NH3 emissions compared to soils amended with only water (Fig S1a). In NH4+ amended desert soils, NH3 emissions averaged 52.5 ± 45.0 µg N–NH3 m−2 h−1 between 0.25 and 12 h, compared to 16.7 ± 10.6 µg N m−2 h−1 in soils amended with only water (Fig S1c).
Discussion
We investigated the dynamics of and mechanisms driving pulsed NO and N2O emissions during drying–wetting cycles in two contrasting drylands. We found that soil NO emissions increased in proportion to the amount of NH4+ added in both sites, although this relationship was weaker in the desert, partially supporting the hypothesis that increasing biological process rates would increase N emissions and suggesting that nitrification may control NO emission magnitude in these coarse-textured soils. In contrast, increasing N supply did not increase N2O emissions at either site, which does not support the hypothesis that N2O emissions are limited by NO3− or NH4+. While N addition did not stimulate N2O emissions, N2O was produced in part by the near-instantaneous reduction of NO3−, raising questions as to the mechanisms driving NO3− reduction in these dryland soils and how factors controlling these emissions could help explain variation in N emissions across ecosystems.
N2O emissions: controls and dynamics
While we expected N2O to increase within minutes after wetting to produce large emission pulses (Eberwein et al. 2020), the incorporation of 15N–NO3− tracer into N2O within 15 min of adding water was unexpected (Fig. 2a,b)—denitrification is an anaerobic process not thought to dominate in well-aerated coarse-textured soils during dry summer months (Werner et al. 2014). Possibly, rapid onset of microbial respiration (Birch 1958; Jenerette and Chatterjee 2012) consumed sufficient O2 to stimulate N2O production via denitrification immediately after adding water, or soil aggregates may have sustained a viable denitrifier population within anoxic microsites throughout the hot and dry summer (Sexstone et al. 1985). Indeed, laboratory studies show denitrification enzyme activity can be maintained in dry soils (Peterjohn 1991; Parker and Schimel 2011), perhaps suggesting this process is viable in deserts. However, because NO is produced as an obligate intermediate during denitrification, and our 15NO3− tracer was not incorporated into NO within 15 min post-wetting in the desert (Fig. 4a), denitrification may not have contributed to rapid N2O emissions. Besides biological processes, chemodenitrification can produce N2O (Zhu-Barker et al. 2015; Heil et al. 2016; Harris et al. 2021), but the abiotic reduction of NO3− has only been reported under manipulated laboratory settings (Davidson et al. 2003; Matus et al. 2019) and is yet to be demonstrated to occur in-situ (Colman et al. 2007, 2008). The detection of the 15N–NO3− label in N2O within 15 min of wetting dry soils at our site shows that dryland soils have the capacity to reduce NO3− immediately after wetting and argues for additional work identifying which processes contribute to rapid N2O emissions.
Even though 15N–NO3− was rapidly reduced to N2O, adding more NO3− did not increase the magnitude of pulsed N2O emissions. This suggests that the processes reducing NO3− to N2O are not limited by soil N availability (i.e., the size of the pipe), and that other factors regulate the magnitude of N2O emissions. For example, more 15N–NO3− tracer was reduced to N2O in the desert (Fig. 2; Table S1), where soils had higher pH and warmer temperature compared to the chaparral (Table 1). These soil properties and environmental conditions can determine which N intermediates are released to the atmosphere, potentially explaining variation in the magnitude of N2O emissions between sites. For example, higher pH desert soils may have increased denitrification rates (Knowles 1982), or warmer temperatures in the desert may have favored abiotic reactions that can produce N2O (McCalley and Sparks 2009; Zhu-Barker et al. 2015). Average peak N2O emissions from the desert were slightly higher compared to emissions measured in tropical forests (66.4 ng N–N2O m−2 s−1; Hall and Matson 2003) and temperate agricultural systems (355 ng N–N2O m−2 s−1; Smith et al. 1994), which are thought of as denitrification hotspots. In addition to differences in pH and temperature between sites, variation in soil properties underneath each shrub could override any effect of experimental N addition on N2O emissions. Indeed, N2O emissions are notoriously difficult to predict since they are often driven by high rates of microbial activity within microsites where soil C and N are concentrated (Sey et al. 2008; Harris et al. 2021). As such, greater replication may be needed to detect effects of N addition over the inherent variability in N2O emissions. Despite this variation, documenting the rapid reduction of NO3− to form N2O is an important step in identifying controls over dryland N2O emissions.
NO emissions: controls and dynamics
Nitrification produced NO at our sites as supported by the detection of 15N–NH4+ in NO (Fig. 4c,d) and the positive response of NO emissions to adding NH4+ (Fig. 6c,d). In addition to nitrification, denitrification also produced NO at both sites; 15N–NO3− was reduced to NO 12 h after wetting dry soils in the desert, and within 15 min in the chaparral (Fig. 4a,b). Denitrifiers can initiate NO3− reduction within hours of decreasing soil O2 concentrations (Liu et al. 2019) and maintain this activity once aerobic conditions return (Roco et al. 2016). We also observed a simultaneous decrease in *[δ18O]NO and *[δ15N]NO over the course of the incubation at both sites, perhaps suggesting other processes produced NO (Fig. 5). While changes to *[δ18O]NO and *[δ15N]NO could have been caused by interactions between NO and VOCs (Walters and Michalski 2016), these observations may also indicate nitrifier denitrification activity as observed in a Mediterranean grassland (Homyak et al. 2016). Nitrifier denitrification produces NO from NO2−, which contains O from both water and air, whereas nitrification produces NO from NH2OH, which contains only O from air (Andersson and Hooper 1983; Buchwald et al. 2012; Medinets et al. 2015; Boshers et al. 2019). As such, the change in *[δ18O]NO may reflect incorporation of 18O from the NO2− produced prior to and after wetting these dry soils (Homyak et al. 2016). Furthermore, biological NO production pathways—including nitrifier denitrification and nitrification—fractionate against 15N by 28–60 ‰ (Robinson 2001), consistent with the simultaneous decrease in *[δ15N]NO observed throughout the incubation. Abiotic reactions may have also contributed to soil NO efflux by converting nitrification intermediates—such as NO2− or NH2OH—to NO (McCalley and Sparks 2009; Heil et al. 2016; Homyak et al. 2017). Regardless of the mechanism, our work suggests that multiple pathways, including those requiring anaerobic conditions, produce NO after wetting these dry coarse-textured soils.
Soil NO-producing pathways were likely limited by soil N availability, since adding more N was associated with higher NO emissions. The positive response of cumulative NO emissions to adding NH4+ is consistent with N limitation of N trace gas production via nitrification (Davidson et al. 2000; Vourlitis et al. 2015; Prosser et al. 2019), as has been observed in other drylands (Hartley and Schlesinger 2000; Eberwein et al. 2020). However, other factors besides N limitation likely contributed to the magnitude of the NO pulse since NO emissions diverged between sites; the tracers were reduced to NO more quickly in the chaparral (Fig. 4), while cumulative NO emissions had a larger positive relationship with NH4+ addition in the desert (Fig. 6). These differences between sites may be explained by background microbial activity. For example, chaparral soils were exposed to fog (Table 1) and were already producing NO before we added water, whereas desert soils were not (Fig. 3b,d). In this sense, non-rainfall water inputs via fog (McHugh et al. 2015) may have influenced the magnitude of pulsed N emissions by resuscitating microbes and priming them for the N we added, helping to explain the rapid NO emission pulse (Fig. 3b,d) and the rapid incorporation of 15N–NO3− into NO (Fig. 4b). In contrast to chaparral, microorganisms in the relatively drier desert took hours to activate before producing the more delayed, but relatively long-lasting, NO emission pulse (Fig. 3a,c). In the desert, we measured higher NH3 emissions relative to the chaparral (Fig S1), consistent with higher soil pH favoring NH3 production from the equilibrium between NH3 and NH4+ (pKa = 9.25; Avnimelech and Laher 1977). This suggests the longer NO emission pulse in the desert could have been sustained by greater NH3 diffusion through soil pore space and supply to nitrifiers even as drying soils may have limited nitrifier access to NH4+ in soil pore water (Stark and Firestone 1995). The role of NH3 diffusion to nitrifiers may also help explain why the relationship between NH4+ addition and NO emissions was weaker in the desert; variable background NH4+ concentrations may have supplied NH3 to nitrifiers even when little N was added to soils. Taken together, our observations support the hypothesis that wetting-induced NO emissions are limited by soil N availability but suggest that environmental and edaphic factors contribute to variation in NO production among ecosystems.
Conclusion
We demonstrate that rapid NO3− reduction (within 15 min) can occur even in coarse summer-dry desert soils under temperature extremes to produce N2O. However, the N2O emissions produced were insensitive to experimentally adding N. Identifying the processes that govern the rapid NO3− reduction pathway will help constrain variation in N emissions across dryland soils as these ecosystems expand with expected changes in climate (Huang et al. 2016). In contrast to N2O, NO emissions were governed by N limitation of multiple N cycling processes, suggesting that N-limited NO production pathways may increase in response to higher rates of atmospheric N deposition (Fenn et al. 2006). These wetting induced N trace gas production pathways appear widespread across ecosystems that experience repeated drying–wetting cycles and will likely become increasingly important sources of atmospheric NO and N2O as global precipitation regimes become more variable.
Data availability
The datasets generated during this study are available in the Dryad repository, https://doi.org/10.6086/D1C39X.
Code availability
Custom code used for calculating trace gas fluxes is available at https://github.com/handr003/TraceGasArray
References
Andersson KK, Hooper AB (1983) O2 and H2O are each the source of one O in NO2- produced from NH3 by Nitrosomonas: 15N-NMR evidence. FEBS Lett 164:236–240. https://doi.org/10.1016/0014-5793(83)80292-0
Avnimelech Y, Laher M (1977) Ammonia volatilization from soils: equilibrium considerations. Soil Sci Soc Am J 41:1080–1084. https://doi.org/10.2136/sssaj1977.03615995004100060013x
Birch HF (1958) The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 10:9–31. https://doi.org/10.1007/BF01343734
Boshers DS, Granger J, Tobias CR, Bohlke JK, Smith RL (2019) Constraining the oxygen isotopic composition of nitrate produced by nitrification. Environ Sci Technol 53:1206–1216. https://doi.org/10.1021/acs.est.8b03386
Buchwald C, Santoro AE, McIlvin MR, Casciotti KL (2012) Oxygen isotopic composition of nitrate and nitrite produced by nitrifying cocultures and natural marine assemblages. Limnol Oceanogr 57:1361–1375. https://doi.org/10.4319/lo.2012.57.5.1361
Caranto JD, Lancaster KM (2017) Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase. Proc Natl Acad Sci USA 114:8217–8222. https://doi.org/10.1073/pnas.1704504114
Casciotti KL, Bo JK, Mcilvin MR, Mroczkowski SJ (2007) Oxygen isotopes in nitrite: analysis, calibration, and equilibration. 79:2427–2436. https://doi.org/10.1021/ac061598h
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C (2013) Climate change 2013: the physical science basis. In: Tignor K, Allen M, Boschung SK, Nauels J, Xia A, Bex Y, Midgley V, et al (eds) Contribution of Working Group I to the fifth assessment report of the intergovernmental panel on climate change.
Colman BP, Fierer N, Schimel JP (2007) Abiotic nitrate incorporation in soil: is it real? Biogeochemistry 84:161–169. https://doi.org/10.1007/s10533-007-9111-5
Colman BP, Fierer N, Schimel JP (2008) Abiotic nitrate incorporation, anaerobic microsites, and the ferrous wheel. Biogeochemistry 91:223–227. https://doi.org/10.1007/s10533-008-9281-9
Coplen TB, Qi H, Révész K, Casciotti K, Hannon JE (2012) Determination of the δ15N and δ18O of nitrate in water; RSIL lab code 2900, Chap 17. In: Révész K, Coplen TB (eds) Stable isotope-ratio methods, Sec C, Methods of the reston stable isotope laboratory: U.S. Geological Survey techniques and methods, Book 10, p. 35.
Coughlin JG, Yu Z, Elliott EM (2017) Efficacy of passive sampler collection for atmospheric NO2 isotopes under simulated environmental conditions. Rapid Commun Mass Spectrom 31:1211–1220. https://doi.org/10.1002/rcm.7885
Crutzen PJ (1979) the Role of NO and NO2 in the chemistry of the troposphere and stratosphere. Ann Rev Earth Planet Sci 7:443–472
Dahal B, Hastings MG (2016) Technical considerations for the use of passive samplers to quantify the isotopic composition of NOx and NO2 using the denitrifier method. Atmos Environ 143:60–66. https://doi.org/10.1016/j.atmosenv.2016.08.006
Dai A (2013) Increasing drought under global warming in observations and models. Nat Clim Chang 3:52–58. https://doi.org/10.1038/nclimate1633
Davidson EA, Chorover J, Dail DB (2003) A mechanism of abiotic immobilization of nitrate in forest ecosystems: the ferrous wheel hypothesis. Glob Chang Biol 9:228–236. https://doi.org/10.1046/j.1365-2486.2003.00592.x
Davidson EA, Keller M, Erickson HE, Verchot LV, Veldkamp E (2000) Testing a conceptual model of soil emissions of nitrous and nitric oxides. Bioscience 50:667. https://doi.org/10.1641/0006-3568(2000)050[0667:TACMOS]2.0.CO;2
Davidson EA, Vitousek PM, Matson PA, Riley R, García-Méndez G, Maass JM (1991) Soil emissions of nitric oxide in a seasonally dry tropical forest of México. J Geophys Res 96:15439. https://doi.org/10.1029/91jd01476
Eberwein JR, Homyak PM, Carey CJ, Aronson EL, Jenerette GD (2020) Large nitrogen oxide emission pulses from desert soils and associated microbiomes. Biogeochemistry 149:239–250. https://doi.org/10.1007/s10533-020-00672-9
Fenn M, Baron JS, Allen EB, Rueth HM, Nydick KR, Geiser L, Bowman WD, Sickman JO, Meixner T, Johnson DW, Neitlich P (2006) Ecological effects of nitrogen deposition in the western United States. Bioscience 53:404. https://doi.org/10.1641/0006-3568(2003)053[0404:eeondi]2.0.co;2
Firestone MK, Davidson EA (1989) Microbiological basis of NO and N2O production and consumption in soil. In: Exchange of trace gases between terrestrial ecosystems and the atmosphere, pp 7–21
Hall SJ, Matson PA (2003) Nutrient status of tropical rain forests influences soil N dynamics after N additions. Ecol Monogr 73:107–129
Harris E, Diaz-Pines E, Stoll E, Schloter M, Schulz S, Duffner C, Li K, Moore KL, Ingrisch J, Reinthaler D, Zechmeister-Boltenstern S, Glatzel S, Brüggemann N, Bahn M (2021) Denitrifying pathways dominate nitrous oxide emissions from managed grassland during drought and rewetting. Sci Adv 7:eabb7118. https://doi.org/10.1126/sciadv.abb7118
Hartley AE, Schlesinger WH (2000) Environmental controls on nitric oxide emission from northern Chihuahuan desert soils. Biogeochemistry 3:279–300. https://doi.org/10.1023/A:1006377832207
Heil J, Vereecken H, Brüggemann N (2016) A review of chemical reactions of nitrification intermediates and their role in nitrogen cycling and nitrogen trace gas formation in soil. Eur J Soil Sci 67:23–39. https://doi.org/10.1111/ejss.12306
Homyak PM, Blankinship JC, Marchus K, Lucero DM, Sickman JO, Schimel JP (2016) Aridity and plant uptake interact to make dryland soils hotspots for nitric oxide (NO) emissions. Proc Natl Acad Sci 113:E2608–E2616. https://doi.org/10.1073/pnas.1520496113
Homyak PM, Kamiyama M, Sickman JO, Schimel JP (2017) Acidity and organic matter promote abiotic nitric oxide production in drying soils. Glob Chang Biol 23:1735–1747. https://doi.org/10.1111/gcb.13507
Homyak PM, Sickman JO (2014) In fluence of soil moisture on the seasonality of nitric oxide emissions from chaparral soils, Sierra Nevada, California, USA. J Arid Environ 103:46–52. https://doi.org/10.1016/j.jaridenv.2013.12.008
Homyak PM, Vasquez KT, Sickman JO, Parker DR, Schimel JP (2015) Improving nitrite analysis in soils: drawbacks of the conventional 2 M KCl extraction. Soil Sci Soc Am J 79:1237–1242. https://doi.org/10.2136/sssaj2015.02.0061n
Huang J, Yu H, Guan X, Wang G, Guo R (2016) Accelerated dryland expansion under climate change. Nat Clim Change 6:166–1171. https://doi.org/10.1038/nclimate2837
Jenerette GD, Chatterjee A (2012) Soil metabolic pulses: water, substrate, and biological regulation. Ecology 93:959–966. https://doi.org/10.1890/11-1527.1
Knowles R (1982) Denitrification. Microbiol Rev 46:43–70
Liu B, Zhang X, Bakken LR, Snipen L, Frostegård Å (2019) Rapid succession of actively transcribing denitrifier populations in agricultural soil during an anoxic spell. Front Microbiol 10:1–12. https://doi.org/10.3389/fmicb.2018.03208
Liu D, Zhu W, Wang X, Pan Y, Wang C, Xi D, Bai E, Wang Y, Han X, Fang Y (2017) Abiotic versus biotic controls on soil nitrogen cycling in drylands along a 3200 km transect. Biogeosciences 14:989–1001. https://doi.org/10.5194/bg-14-989-2017
Matus F, Stock S, Eschenbach W, Dyckmans J, Merino C, Nájera F, Köster M, Kuzyakov Y, Dippold MA (2019) Ferrous wheel hypothesis: abiotic nitrate incorporation into dissolved organic matter. Geochem Cosmochim Acta 245:514–524. https://doi.org/10.1016/j.gca.2018.11.020
McCalley C, Sparks J (2009) Abiotic gas formation drives nitrogen loss from a desert ecosystem. Science 326(326):837–840. https://doi.org/10.1126/science.1178984
McHugh TA, Morrissey EM, Reed SC, Hungate BA, Schwartz E (2015) Water from air: an overlooked source of moisture in arid and semiarid regions. Sci Rep 5:1–6. https://doi.org/10.1038/srep13767
Medinets S, Skiba U, Rennenberg H, Butterbach-bahl K (2015) A review of soil NO transformation: associated processes and possible physiological significance on organisms. Soil Biol Biochem 80:92–117. https://doi.org/10.1016/j.soilbio.2014.09.025
Parker SS, Schimel JP (2011) Soil nitrogen availability and transformations differ between the summer and the growing season in a California grassland. Appl Soil Ecol 48:185–192. https://doi.org/10.1016/j.apsoil.2011.03.007
Peterjohn WT (1991) Denitrification: enzyme content and activity in desert soils. Soil Biol Biochem 23:845–855
Peterjohn WT, Schlesinger WH (1990) Nitrogen loss from deserts in the southwestern United States. Biogeochemistry 10:67–79. https://doi.org/10.1007/BF00000893
Peterjohn WT, Schlesinger WH (1991) Factors controlling denitrification in a Chihuahuan desert ecosystem. Soil Sci Soc Am J 55:1694–1701. https://doi.org/10.2136/sssaj1991.03615995005500060032x
Placella SA, Firestone MK (2013) Transcriptional response of nitrifying communities to wetting of dry soil. Appl Environ Microbiol 79:3294–3302. https://doi.org/10.1128/AEM.00404-13
Prosser JI, Hink L, Gubry-Rangin C, Nicol GW (2019) Nitrous oxide production by ammonia oxidisers: physiological diversity, niche differentiation and potential mitigation strategies. Glob Chang Biol. https://doi.org/10.1111/gcb.14877
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://R-project.org/
Robinson D (2001) δ15N as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162. https://doi.org/10.1016/S0169-5347(00)02098-X
Roco CA, Bergaust LL, Shapleigh JP, Yavitt JB (2016) Reduction of nitrate to nitrite by microbes under oxic conditions. Soil Biol Biochem 100:1–8. https://doi.org/10.1016/j.soilbio.2016.05.008
Schlesinger WH, Reynolds JF, Cunningham GL, Huenneke LF, Jarrell WM, Virginia RA, Whitford WG (1990) Biological feedbacks in global desertification. Science 247:1043–1048. https://doi.org/10.1126/science.247.4946.1043
Scholes MC, Martin R, Scholes RJ, Parsons D, Winstead E (1997) NO and N2O emissions from savanna soils following the first simulated rains of the season. Nutr Cycl Agroecosyst 48:115–122. https://doi.org/10.1023/A:1009781420199
Sexstone AJ, Revsbech NP, Parkin TB, Tiedje JM (1985) Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci Soc Am J 49:645. https://doi.org/10.2136/sssaj1985.03615995004900030024x
Sey BK, Manceur AM, Whalen JK et al (2008) Small-scale heterogeneity in carbon dioxide, nitrous oxide and methane production from aggregates of a cultivated sandy-loam soil. Soil Biol Biochem 40:2468–2473. https://doi.org/10.1016/j.soilbio.2008.05.012
Sigman DM, Casciotti KL, Andreani M, Barford C, Galanter M, Böhlke JK (2001) A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal Chem 73:4145–4153. https://doi.org/10.1021/ac010088e
Smith KA, Clayton H, Arah JRM, Christensen S, Ambus P, Fowler D, Hargreaves KJ, Skiba U, Harris GW, Wienhold FG, Klemedtsson L, Galle B (1994) Micrometeorological and chamber methods for measurement of nitrous-oxide fluxes between soils and the atmosphere—an overview and conclusions. J Geophys Res-Atmos 99:16541–16548
Soper FM, Boutton TW, Groffman PM, Sparks JP (2016) Nitrogen trace gas fluxes from a semiarid subtropical savanna under woody legume encroachment. Global Biogeochem Cycles 30:614–628. https://doi.org/10.1002/2015GB005298
Stark JM, Firestone MK (1995) Mechanisms for soil-moisture effects on activity of nitrifying bacteria. Appl Environ Microbiol 61:218–221
Van Groenigen JW, Huygens D, Boeckx P et al (2015) The soil n cycle: new insights and key challenges. Soil 1:235–256. https://doi.org/10.5194/soil-1-235-2015
Venterea RT, Rolston DE (2000) Mechanisms and kinetics of nitric and nitrous oxide production during nitrification in agricultural soil. Glob Chang Biol 6:303–316. https://doi.org/10.1046/j.1365-2486.2000.00309.x
von Sperber C, Chadwick OA, Casciotti KL et al (2017) Controls of nitrogen cycling evaluated along a well-characterized climate gradient. Ecology 98:1117–1129. https://doi.org/10.1002/ecy.1751
Vourlitis GL, Defotis C, Kristan W (2015) Effects of soil water content, temperature and experimental nitrogen deposition on nitric oxide (NO) efflux from semiarid shrubland soil. J Arid Environ 117:67–74. https://doi.org/10.1016/j.jaridenv.2015.02.011
Walters WW, Michalski G (2016) Theoretical calculation of oxygen equilibrium isotope fractionation factors involving various NOy molecules, OH, and H2O and its implications for isotope variations in atmospheric nitrate. Geochim Cosmochim Acta 191:89–101. https://doi.org/10.1016/j.gca.2016.06.039
Wang C, Wang X, Liu D, Wu H, Lü X, Fang Y, Cheng W, Luo W, Jiang P, Shi J, Yin H, Zhou J, Han X, Bai E (2014) Aridity threshold in controlling ecosystem nitrogen cycling in arid and semi-arid grasslands. Nat Commun. https://doi.org/10.1038/ncomms5799
Werner C, Reiser K, Dannenmann M, Hutley LB, Jacobeit J (2014) N2O, NO, N2 and CO2 emissions from tropical savanna and grassland of northern Australia: an incubation experiment with intact soil cores. Biogeosciences 11:6047–6065. https://doi.org/10.5194/bg-11-6047-2014
Yu Z, Elliott EM (2017) Novel method for nitrogen isotopic analysis of soil-emitted nitric oxide. Environ Sci Technol 51:6268–6278. https://doi.org/10.1021/acs.est.7b00592
Zhu-Barker X, Cavazos AR, Ostrom NE, Horwath WR, Glass JB (2015) The importance of abiotic reactions for nitrous oxide production. Biogeochemistry 126:251–267. https://doi.org/10.1007/s10533-015-0166-4
Acknowledgements
We are thankful to Eric Slessarev for suggestions on a version of this manuscript. We thank the UC Natural Reserve System (https://doi.org/10.21973/N3V66D) for access to field sites, Beatriz Vindiola and Delores Lucero for their help with the passive samplers, and David Lyons from the UCR Environmental Sciences Research Laboratory for help with sample analyses. We also thank the National Science Foundation (DEB 1916622 and DEB 1656062) for their support.
Funding
This research was funded by the National Science Foundation (DEB 1916622 and DEB 1656062).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Jonathan Sanderman
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krichels, A.H., Homyak, P.M., Aronson, E.L. et al. Rapid nitrate reduction produces pulsed NO and N2O emissions following wetting of dryland soils. Biogeochemistry 158, 233–250 (2022). https://doi.org/10.1007/s10533-022-00896-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-022-00896-x