Abstract
The knowledge of tree species dependent turnover of soil organic matter (SOM) is limited, yet required to understand the carbon sequestration function of forest soil. We combined investigations of 13C and 15N and its relationship to elemental stoichiometry along soil depth gradients in 35-year old monocultural stands of Douglas fir (Pseudotsuga menziesii), black pine (Pinus nigra), European beech (Fagus sylvatica) and red oak (Quercus rubra) growing on a uniform post-mining soil. We investigated the natural abundance of 13C and 15N and the carbon:nitrogen (C:N) and oxygen:carbon (O:C) stoichiometry of litterfall and fine roots as well as SOM in the forest floor and mineral soil. Tree species had a significant effect on SOM δ13C and δ15N reflecting significantly different signatures of litterfall and root inputs. Throughout the soil profile, δ13C and δ15N were significantly related to the C:N and O:C ratio which indicates that isotope enrichment with soil depth is linked to the turnover of organic matter (OM). Significantly higher turnover of OM in soils under deciduous tree species depended to 46% on the quality of litterfall and root inputs (N content, C:N, O:C ratio), and the initial isotopic signatures of litterfall. Hence, SOM composition and turnover also depends on additional—presumably microbial driven—factors. The enrichment of 15N with soil depth was generally linked to 13C. In soils under pine, however, with limited N and C availability, the enrichment of 15N was decoupled from 13C. This suggests that transformation pathways depend on litter quality of tree species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soils in forest ecosystems bear a high potential as carbon (C) sinks in the mitigation of climate change (Pan et al. 2011). Tree species identity plays a crucial role in the C cycle of these ecosystems, e.g. by fueling soil with bio- and necromass, respectively (Augusto et al. 2015). The total amount of C that is stored in the forest floor and mineral soil is affected by the dominating tree species (Mueller et al. 2015). Furthermore, stoichiometric ratios like the carbon:nitrogen (C:N) ratio of soil organic matter (SOM) are influenced by the forest stand (Cools et al. 2014; Lorenz and Thiele-Bruhn 2019). Ecological stoichiometry using elemental ratios is a suitable tool to assess SOM and its turnover (Manzoni et al. 2010; Zechmeister-Boltenstern et al. 2015). For this, the C:N ratio is commonly used (Stevenson 1994). Also the oxygen:carbon (O:C) ratio bears high potential to characterize SOM because it reflects the state of oxidation of SOM (Beyer et al. 1998). Furthermore, the abundance of stable isotopes (13C and 15N) in soils also provide a powerful tool for investigating spatial and temporal SOM dynamics (Ehleringer et al. 2000; Brüggemann et al. 2011; Craine et al. 2015). Variations in the isotopic composition are useful for tracing carbon sources and fluxes between plants, microorganisms and soils, thus serving to elucidate the impact of plant inputs on SOM formation (Balesdent et al. 1987). A combination of both approaches, ecological stoichiometry and stable isotopes, in soil depth gradients promises to get deeper insights into the turnover of SOM. To our knowledge, this has not been used before to characterize the turnover of tree species dependent organic matter (OM) in the soil.
Typically, 13C and 15N show trends of enrichment with increasing soil depth that were related to aging and turnover of OM (Nadelhoffer and Fry 1988; Billings and Richter 2006; Trumbore 2009). Several SOM turnover and stabilization mechanisms were identified that can lead to a variation of the natural abundance of 13C. Litter with lower δ13C values from aboveground plant materials triggers the topsoil, while the contribution of 13C-enriched root inputs to SOM δ13C increases with increasing soil depth (Bird et al. 2003). Root inputs encompass C release from plant roots to soil including: (1) root cap and border cell loss, (2) necromass from root cells and tissues, (3) C flow to root-associated, soil living symbionts (e.g. mycorrhiza), (4) gaseous losses, (5) root exudates, and (6) mucilage (Jones et al. 2009). During the microbial metabolism of C, preferentially 13C-depleted molecules will be respired by microorganisms and the remaining SOM will be 13C-enriched (Lerch et al. 2011). In general, microorganisms are 13C-enriched compared to plant material or bulk SOM (Dijkstra et al. 2006) and the contribution of microbial derived C increases with the extent of OM turnover (Boström et al. 2007). Additionally, OM associated with soil minerals is characterized by increased δ13C values compared to free or occluded light OM fractions (Schrumpf et al. 2013). The association of OM with minerals is an important mechanism for its stabilization in soil (von Lützow et al. 2007). The prevalence of SOC decrease and δ13C increase with depth in well-drained forest soils has prompted the use of the gradient of SOC plotted against δ13C as a proxy for SOM turnover (Acton et al. 2013). Consequently, depth-related interconnection of δ13C and SOC describes the rate of change in 13C natural abundance along a decay continuum from fresh litter inputs to more decomposed SOM (Garten et al. 2000).
The absolute enrichment of 15N over soil depth can be determined as the difference between the maximum enrichment of 15N in the mineral soil and the litter bearing OL horizon (Hobbie and Ouimette 2009). The development of 15N with soil depth is related to N cycling processes that are coupled to SOM turnover (Emmett et al. 1998). Similar to δ13C values, organo-mineral associations (Kramer et al. 2017) and the accumulation of 15N enriched microbial biomass in more transformed SOM (Wallander et al. 2009) can drive the δ15N patterns within soil. Furthermore, the type and degree of mycorrhizal associations (Hobbie and Högberg 2012), enzymatic hydrolysis (Silfer et al. 1992), N losses after ammonification, nitrification and denitrification (Högberg 1997; Pörtl et al. 2007), atmospheric depositions (Vallano and Sparks 2013) and mixing of soil N through bioturbation (Wilske et al. 2015) contributes to the 15N enrichments along the soil profile. Both δ13C and δ15N are mechanistically linked through the decomposition and microbial processing of SOM (Nel et al. 2018), thus highlighting the suitability of both parameters to determine the degree of organic matter turnover in soil.
In natural mixed forest ecosystems it is difficult to track down a tree species effect on SOM status; therefore common garden experiments, where different tree species were planted in adjacent blocks at the same time on similar soil, were established to study tree species effects (Reich et al. 2005; Vesterdal et al. 2013). Important insights into the relationship between tree species and the cycling of soil C and other nutrients in forest ecosystems were gained from common garden experiments (e.g. Mueller et al. 2012; Gurmesa et al. 2013). Nevertheless, such experiments are often handicapped by a previous land-use conversion, e.g. from arable land or from clear felled forests (Vesterdal et al. 2008). Old SOM from former land-use types often makes the interpretation of the effects of tested species and their SOM on SOM dynamics rather difficult (Balesdent et al. 2018). Callesen et al. (2013) revealed in a common garden experiment that the patterns of δ15N in soil profiles reflected the former arable land-use type. Comparable with common garden experiments, differently afforested soils at post-mining sites provide a unique opportunity for understanding mechanisms in SOM formation (Frouz et al. 2009). In particular, sites that are free from old C sources can be suitable but investigations on such sites are rare. Due to this, further research is required to clarify if δ13C and δ15N, and thus the SOM status in organic forest floor horizons (litter—OL, fragmented—OF, humified—OH) and mineral soil differs between tree species.
This research was conducted on a post-mining site, where previous accumulation of plant or coal material are negligible (Lorenz & Thiele-Bruhn 2019). We studied monocultural stands of Douglas fir (Pseudotsuga menziesii), black pine (Pinus nigra), European beech (Fagus sylvatica) and red oak (Quercus rubra) that were grown for 35 years under identical soil and geomorphological conditions to assess tree species effects on the SOM status. In more detail, we investigated the natural abundance of 13C and 15N in combination with C:N and O:C stoichiometry of litterfall and root inputs (determined as belowground phytomass) as well as SOM in depth gradients of forest soils to answer the following questions:
-
(1)
Do litterfall and root inputs differ in their isotopic signatures of δ13C and δ15N between tree species?
-
(2)
Is there a tree species effect on δ13C and δ15N in the depth gradients starting from the OL horizon down to 10–30 cm of mineral soil?
-
(3)
Are stable isotope contents in the depth profiles related to the stoichiometry (C:N and O:C ratio) of the bulk soil?
-
(4)
Varies the decomposition of OM and the stabilization of it in soil significantly between tree species and if yes, are litterfall and/or root properties important for these processes?
Materials and methods
Study site
The study was conducted at the afforested spoil heap ‘Sophienhöhe’, located in the northwest of the lignite open-cast mine ‘Hambach’ in the Rhineland, Germany (N 50° 56.11′, E 6° 26.56′). There, boundary conditions regarding soil, climate, topography and management were highly similar, equivalent to a common garden experiment. The Regosols at the investigated sites developed on the same sandy gravelly parent material (Lorenz and Thiele-Bruhn 2019). The carbonate-free parent material that was used for the spoil heap recultivation had a C content of 0.20 ± 0.05% and a C/N molar ratio of 7.5 ± 1.2 (Table S1). Therefore, a relevant impact of old or fossil carbon from former land use types and the introduction of coal from lignite mining was excluded (Lorenz and Thiele-Bruhn 2019). The investigation was carried out in monocultural stands of Douglas fir (Pseudotsuga menziesii), black pine (Pinus nigra), European beech (Fagus sylvatica) and red oak (Quercus rubra) that were afforested in 1982 on the western slopes of the spoil heap. Within 35 years after the start of the afforestation organic layers had developed that were classified as Moder (Zanella et al. 2018) with slight differences between tree stands. Dependent on the thickness of the OH layer, Dysmoder was the dominant humus form that coexisted in some patchy sections with Eumoder under Douglas fir, beech and oak, while under pine solely Dysmoder had developed (Lorenz and Thiele-Bruhn 2019).
Sampling scheme and sample preparation
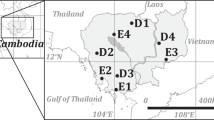
Each species stand is subdivided in six to ten plots with a size of 1780 ± 660 m2 by skid trails established in slope line. For each of the four stands, five plots were selected for sampling of the forest floor, mineral soil, roots and litterfall (Fig. 1). Sampling points (light grey circles) within one plot were located at least 25 m away from forest roads and in the upper parts of the middle slopes. In April 2016, forest floor samples were taken with a steel frame (20 cm × 20 cm) and carefully separated into the organic litter, fragmented, and humified horizon, OL, OF and OH, respectively, according to Zanella et al. (2018). Afterwards, bulk soil samples were taken at three different depths (0–5 cm, 5–10 cm, 10–30 cm) from excavated 50 cm × 50 cm × 50 cm pits. To ensure representativeness, forest floor and soil samples (grey rectangles) were taken from four positions and samples from similar depths were subsequently pooled. In total 60 forest floor samples and 60 mineral soil samples were collected (five per depth in each stand, Table S2), transported and stored at 4 °C for further preparation. Forest floor samples were dried at 60 °C and visible roots were carefully sorted out. Mineral soil samples were passed through a 2 mm sieve, roots were removed and the soil samples were dried at 60 °C. All samples were ground and homogenized using a ball mill (Retsch MM400, Retsch GmbH, Haan, Germany).
Sampling design at the study site ‘Sophienhöhe’. 35 year-old afforested monocultural stands of Douglas fir (Pseudotsuga menziesii), black pine (Pinus nigra), European beech (Fagus sylvatica) and red oak (Quercus rubra) were investigated. They are located on the western exposed slopes (inclination: 22.2° ± 2.2°) of the spoil heap. Each stand is subdivided in six to ten plots with a size of 1780 ± 660 m2 by skid trails established in slope line. For each of the four tree stands, five plots were selected for sampling (light grey circles) of the forest floor (grey rectangles), mineral soil (grey rectangles), roots (dark grey circles) and litterfall (litter trap)
We performed root sampling 2 years later, in April 2018 and distinguished roots from different horizons. To do so, we collected five replicate samples of forest floor and mineral soil (dark grey circles in Fig. 1) with a distance of 1 m around a tree within each of the five plots per species stand. Forest floor roots were collected using a steel frame (20 cm × 20 cm). Underneath, mineral soil roots were collected using a root auger with a diameter of 8 cm (Eijkelkamp Soil & Water, Giesbeck, Netherlands). The cores of mineral soil were divided into the three subsamples (0–5 cm, 5–10 cm. 10–30 cm). In total, we collected 400 root samples (25 per depth in each species stand, Table S2) that were transported and stored at 4 °C. In the laboratory, the forest floor samples were spread out in plastic bowls and roots were carefully separated using a tweezer. The roots were carefully washed to remove adherent soil particles. The mineral soil samples were put into plastic bowls and immediately washed with water to separate roots. Roots with a diameter ≤ 5 mm were dried at 105 °C to determine dry weights and a subset of 80 samples (five per depth per species) was homogenized using a ball mill (Retsch MM400, Retsch GmbH, Haan, Germany) for further chemical analysis.
In each of the five plots per species stand the litterfall was collected using litter traps made with nylon mesh (0.5 mm mesh size) that was fixed on a wooden frame (1 m × 1 m). Litter traps were installed 1 m above the soil surface and located in the plot near the central soil sampling point (Fig. 1). In the timespan from July 2016 to June 2017 the litter traps were monthly emptied. In the laboratory, the 240 samples were immediately separated into foliar and non-foliar fractions and dried at 60 °C to determine dry weights (Ukonmaanaho et al. 2016). The foliar fraction of the 12 monthly samples of each litter trap were pooled into one mixed sample. Consequently, five litterfall samples per tree species resulted in a total number of 20 litterfall samples (Table S2) that were homogenized using a ball mill (Retsch MM400, Retsch GmbH, Haan, Germany) for further chemical analysis.
Laboratory analysis
Total contents of C, N and O were determined using an Elemental Analyser EA3000 (HEKAtech GmbH, Wegberg, Germany). Soil samples were acidic and free of carbonate (Lorenz and Thiele-Bruhn 2019), thus the measured total C content represents organic C. The contents of the elements were used to calculate the molar C:N and O:C ratios. The stable isotopes 13C and 15N were determined by an IsoPrime 100 isotope ratio mass-spectrometer (IsoPrime Corporation, Cheadle, UK) and vario ISOTOPE cube elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). Stable isotope compositions are reported in delta notation (δ13C ‰ and δ15N ‰) relative to Vienna Pee-Dee Belemnite (VPDB) for C, using the international reference materials IAEA-CH-7 (− 32.151‰ VPDB SD ± 0.05‰) as a standard, and relative to atmospheric N2 for N, using IAEA-N-1 (+ 0.4‰ air N2 SD ± 0.2‰), IAEA-N-2 (+ 20.3‰ air N2 SD ± 0.2‰) and USGS32 (+ 180‰ air N2 SD ± 1‰) as standard according to Eq. (1):
where R represents the ratio of 13C/12C or 15N/14N, respectively. The measurement error of δ15N was approximately 0.2‰ and < 0.1‰ for δ13C.
Data processing and statistics
The relationship between the prevalent vertical decrease of SOC and increase of δ13C in depth profiles was used as a natural indicator of SOC turnover (Acton et al. 2013). The slope (β) of the linear regression (y = a + βx) between the mean δ13C values and their respective log-transformed C concentrations (mg C g−1) was calculated and is referred as βδ13C value. The distribution of 15N along soil depth profiles was compared between tree stands using the soil enrichment factor (εsoil15N). It is defined as absolute enrichment between the OL horizon and the 10–30 cm mineral soil layer (Hobbie and Ouimette 2009) and was calculated following Eq. (2):
The following statistical analyses were conducted separately for litter inputs (litterfall and roots) and each depth starting from the OL horizon down to 10–30 cm with the R statistical package version 3.3.2. (R Core Team 2016). Boxplots and one-way analysis variance (ANOVA) as pretests were carried out to inspect the data structure. The residuals of ANOVA were tested for normality and homoscedasticity using the Shapiro–Wilk test respectively Levene’s test. Accordingly, normal distributed and homoscedastic data were tested for significant differences between tree species by one-way ANOVA followed by the Tukey’s honest significant difference (HSD) post hoc test. Significant differences between tree species for normal distributed but heteroscedastic data were tested using Welch-ANOVA followed by a pairwise t test with Bonferroni–Holm correction. In case data was not normal distributed but homoscedastic the Kruskal–Wallis test was applied followed by the Dunn test. Variance analyses and necessary pretests were performed with a significance level of p < 0.05. The results are presented in arithmetic mean ± standard deviation (SD) for the different tree stands. To characterize relationships between isotopic (δ13C, δ15N,) and stoichiometric (C:N, O:C) parameters regression analyses with linear and logarithmic functions were done. Additionally, multiple linear regression models were generated to analyze explaining variables for βδ13C and εsoil15N values. For this purpose, C, N, C:N, O:C, δ13C, δ15N, as well as the biomass of litterfall and roots were used as independent variables. To simplify the complexity of the model, parameters were stepwise eliminated that decrease the quality of the regression model by assessing R2 and p values. Finally, the most appropriate models that comprise only parameters with significant portions of the explainable variance were used to discuss driving factors for βδ13C and εsoil15N values. The results of the regression analyses were described as significant in cases where p < 0.05.
Results
Litter inputs
The total annual foliar litterfall in pine stands was significantly higher compared to beech stands, whereas under Douglas fir and oak intermediate amounts were reached (Table 1). The 13C content of litterfall were significantly highest in Douglas fir stands and declined in the sequence Douglas fir > pine ≥ oak > beech. Also, δ15N of litterfall was significantly higher in Douglas fir stands compared to the other tree species. The litterfall of coniferous species was characterized by significantly higher C:N ratios compared to deciduous forest stands. The O:C ratio of litterfall decreased in the order oak > beech > pine > Douglas fir.
In contrast to the litterfall, substantially and in part significantly higher root biomasses were detected in the upper 30 cm of soil under deciduous tree species than under conifers (Table 1). In general, the roots of all tree species were significantly enriched in 13C by 4.56 ± 0.99‰ and 15N by 3.01 ± 0.61‰ compared to the litterfall. Douglas fir roots were characterized by significantly higher δ13C values compared to those of pine and beech, while the significantly lowest δ13C values were determined in oak roots. Similar differences between tree stands were found for δ15N of roots as well as for litterfall δ15N. Obviously, the isotopic signatures of roots in the forest floors did not differ from roots in deeper soil horizons but the tree species effect was similarly pronounced in each soil depth. In contrast to the litterfall, the C:N ratio of beech and oak roots was significantly higher compared to the coniferous species (Table S3). The O:C ratio of roots was highest in the oak stand similar to the litterfall.
Depth profiles of bulk soil δ13C and δ15N and their relationship to stoichiometry patterns
The parent material for soil recultivation was characterized by a δ13C value of − 29.69 ± 0.13‰ and a δ15N value of − 0.89 ± 0.09‰ (Table S1). In general, with increasing soil depth an enrichment of 13C and 15N was observed, while the contents of C and N decreased (Fig. 2). A small deviation from this pattern occurred for 13C in the forest floor horizons, where a depletion or no significant variation from OL to OH horizon was detected. δ13C varied in a range from − 29.37‰ (oak, OF) to − 26.19‰ (Douglas fir, 10–30 cm). The coniferous species Douglas fir and pine caused significantly higher δ13C values in the forest floor compared to beech and oak. In the mineral soil lowest δ13C values were found in the oak stands (− 28.13‰ to − 27.32‰), while in the Douglas fir stands highest δ13C values from − 26.91‰ to − 26.19‰ were measured (Table S4). Throughout the soil profile, a significant effect of tree species on δ13C was detected (Fig. 2b).
Depth gradients of C (a), δ13C (b), N (c) and δ15N (d) from the OL horizon to 10–30 cm. Coniferous species Douglas fir (“D”) and pine (“P”) are presented by black symbols and the deciduous species beech (“B”) and oak (“O”) by grey symbols. Significant differences between tree species are marked with “*” (p < 0.05), “**” (p < 0.01), “***” (p < 0.001). Detailed information about statistical differences can be found in Table S4
Compared to δ13C, δ15N varied in a wider range from − 6.93‰ (pine, OL) to 0.54‰ (beech, 10–30 cm) and depth gradients were more pronounced. The forest floor horizons (OL, OF, OH) of Douglas fir showed significantly higher δ15N values compared to the other tree species (Fig. 2d). In the first two mineral soil layers (0–5 cm, 5–10 cm) the δ15N values of all tree species converged, while at a depth from 10 to 30 cm under beech and Douglas fir significantly higher values were determined compared to oak. Consequently, a tree species effect on δ15N was found in the forest floor as well as the deepest investigated mineral soil layer from 10 to 30 cm.
Regression analyses revealed that δ13C and δ15N were related to the C:N and O:C ratio (Table 2). The relationships of these two stoichiometric ratios of the bulk soil were stronger with δ15N than with δ13C and were better described by a logarithmic equation rather than by a linear equation (Tables 2 and S5). Along the soil profile from OL to 10–30 cm depth the C:N ratio decreased in a range from 52.8 (oak, OL) to 15.7 (beech, 10–30 cm), while the O:C ratio increased from 0.40 (Douglas fir, pine, OL) to 3.07 (beech, 10–30 cm) (Table S4). With increasing soil depth the decline of C:N was exponentially correlated to an enrichment of 15N (Fig. 3a). Different slopes of the regression lines showed that the relationship between C:N and δ15N was differently pronounced dependent on the tree species. Similarly close regressions were determined for the relationship between O:C and δ15N (Fig. 3b). Yet, curves exponentially increased, showing 15N enrichment with increasing O:C ratio.
Relationship between δ15N and the molar ratios of C:N and O:C. Douglas fir (“D”) and pine (“P”) are presented by blue and green symbols, beech (“B”) and oak (“O”) are represented by yellow and red symbols. Detailed information about statistics of the logarithmic and linear relationships can be found in Table 2 and Table S5
βδ13C values and εsoil15N and the contribution of litterfall and root inputs
The βδ13C values for beech (− 1.14) were significantly more negative compared to pine (− 0.64) and Douglas fir (− 0.81) by a factor of 1.8, 1.4 respectively (Fig. 4a). For the oak stands intermediate βδ13C values (− 0.87) were determined. Values for εsoil15N differed up to a factor of 1.7 with significantly higher values under beech (7.02‰) and pine (6.52‰) compared to oak (4.76‰) and Douglas fir (4.05‰) (Fig. 4b). Apparently, βδ13C values differed systematically between coniferous and deciduous species, while εsoil15N depended more on individual tree species.
Using multiple linear regression analyses, the impact of litterfall and root properties on both indices, βδ13C and εsoil15N, was assessed. In total, 49% and 74% of the variability in βδ13C and εsoil15N was represented by the explaining variables (Table 3). Higher βδ13C values were associated with litterfall that was characterized by higher δ13C values and lower δ15N values. Furthermore, root C:N played a significant role for βδ13C. Litterfall with lower C:N ratios and more negative δ15N values were related to higher εsoil15N. Additionally, higher root δ13C values and lower O:C ratios of roots were associated with higher εsoil15N.
Discussion
Isotopic signatures of litter inputs
In all investigated forest stands roots were consistently enriched in 13C and 15N compared to the litterfall (Table 1). Several post-photosynthetic allocation mechanisms can lead to an enrichment of 13C in heterotrophic plant organs compared to leaves (Cernusak et al. 2009). For example, a greater allocation of depleted C to lignin and lipid pools and an export of less depleted carbohydrates to roots result in an enrichment of 13C in belowground organs (Hobbie and Werner 2004; Badeck et al. 2005). The observed significant impact of tree species on root and litterfall δ13C is caused by a complex interplay of physiological differences between the tree species and their response to environmental conditions, which has been thoroughly reviewed by Dawson et al. (2002). The higher δ13C values of the here investigated coniferous species in comparison to deciduous trees (Table 1) are mainly caused by a higher intrinsic water-use-efficiency, lower stomatal conductance and lower photosynthetic rates (Brooks et al. 1997). It must be noted that the δ13C of different plant parts varies on diurnal, seasonal and annual to interannual time scales (Brüggemann et al. 2011). Here we use the δ13C of the annual litter inputs as reference points to evaluate the decomposition of OM along soil profiles (Bowling et al. 2008).
The generally lower δ15N values of litterfall compared to roots are in line with findings of other studies (Högberg et al. 1996; Templer et al. 2007). This pattern can be assigned to fractionation during N transformation and transport within the plant that leads to an assimilation of 15N-depleted N in leaves and 15N-enriched N in roots (Pardo et al. 2013). Moreover, the formation of mycorrhizal symbioses is one of the most important factors influencing the δ15N signature of leaves. The here investigated tree species Douglas fir, pine, beech and oak are well known to form symbioses with ectomycorrhizal (EM) fungi (Wang and Qiu 2006). 15N-enriched N compounds are preferentially retained by the fungal biomass, while 15N-depleted N compounds are transported to their host plant (Craine et al. 2009). The biggest difference between root and litterfall δ15N was determined for beech (3.69 ± 0.96‰) followed by oak (3.33 ± 0.52‰). This range is in agreement with differences of ~ 4‰ observed by Hobbie and Colpaert (2003). According to them, the amount of ectomycorrhizal mass included with the roots also determines the enrichment of roots in 15N compared to foliar tissues. The threefold higher root biomass of beech and oak compared to Douglas fir and pine (Table 1) can therefore be responsible for the highest differences between the plant organs at these stands. Analyzing the abundance of mycorrhizal fungi in symbioses with the investigated tree stands was beyond the scope of the study. However, it is reported that the EM fungal biomass does not vary significantly between beech and conifers in temperate forests but the mechanisms behind the regulation of EM fungal biomass are highly complex (Awad et al. 2019). Additional to mycorrhizal associations, the variability in plant δ15N depends on the form of soil N that plants predominantly acquire (Vallano and Sparks 2013). Denitrification and nitrification both discriminate against 15N because 15N-depleted nitrate can be leached from the soil, resulting in 15N-enrichment of the remaining N that can be taken up by plants (Hobbie and Högberg 2012). High nitrate concentrations of soils under Douglas fir (Zeller et al. 2019) can account for the significantly highest litterfall δ15N values at the study site ‘Sophienhöhe’. Beech, with the second highest δ15N values for litter inputs, is recognized as a tree species that promotes nitrification in soils (Andrianarisoa et al. 2010). However, a more profound investigation of specific N cycling processes in the plant-soil system that potentially influence the natural abundance of δ15N is beyond the scope of this study.
Depth profiles of δ13C and δ15N and their relationship to stoichiometry patterns
Within 35 years after afforestation distinct depth profiles of δ13C developed in all investigated forest stands confirming findings of Brunn et al. (2017), who demonstrated that three decades after afforestation are sufficient to yield such profiles. The gradients from OL to OH in our investigated forest stands were characterized by a decrease or at least no alteration of δ13C (Fig. 2b). Within the early stages of OM decomposition water-soluble substances and non-lignified carbohydrates are degraded, while the proportion of lignin residually increases (Berg 2008; Osono et al. 2008). Lignin is characterized by lower δ13C values compared to bulk foliar δ13C, while cellulose and sugars are characterized by higher values (Bowling et al. 2008). Therefore, a selective preservation of lignin, and lignin building blocks, respectively (Suárez-Abelenda et al. 2015), which cannot be attacked by the vast majority of decomposers, can lead to the depletion of 13C downwards through the organic horizons under Douglas fir, pine and oak. No significant depletion of 13C was found under beech. It appears that beech maybe belongs to the group of tree species, where the carbohydrate-dominated early stage of litter decomposition is so marginal that it has no measurable impact on δ13C values (Berg and McClaugherty 2014). The fact that the sampling campaign was in April and the litterfall predominately occurred in October and November corroborates the assumption. The time difference between litterfall and organic-layer sampling can also be responsible for the high extent of 13C enrichment from litterfall to OL material. In the mineral soil horizons, however, δ13C of bulk SOM increased with increasing depth. A relevant contribution of atmospheric 13C-depleted CO2 (Francey et al. 1999), to OM at the soil surface can be excluded because the 35 years old afforested sites are rather young. Instead, 13C-depleted litter from aboveground plant materials accumulates at the soil surface, while the contribution of OM that derives from 13C-enriched roots to SOM formation increases with soil depth (Bird et al. 2003). Correspondingly, in our study roots were on average higher with 13C by 4.56 ± 0.99‰ compared to the litterfall. Furthermore, the kinetic fractionation of C isotopes during the maturation of SOM leads to an enrichment of 13C with increasing depth (Wynn et al. 2006). Within the microbial metabolism of C sources preferentially 13C-depleted CO2 is respired by microorganisms, while the remaining SOM including the soil microbial biomass becomes enriched in 13C (Werth and Kuzyakov 2010). Thus, microorganisms fractionate during the C assimilation and/or preferentially use 13C-enriched substrates (Schwartz et al. 2007). Especially in mineral soils of forests 13C-enriched microbial-derived OM has a larger share of bulk SOM δ13C values than lignin or aliphatic biopolymers (Dümig et al. 2013). Throughout the soil profile, δ13C of SOM was affected by tree identity with consistently highest values in Douglas fir stands and lowest values in oak stands. In contrast, Marty et al. (2015) found a negative impact of the percentage of conifers in Canadian forests on δ13C values in mineral horizons. They assume that this was caused by lower microbial activity and/or lower SOM degradation at sites dominated by conifers. Yet, in our study the differences in the SOM δ13C values among tree species reflect the isotopic signatures of the OL horizon that in turn strongly correlated with litterfall δ13C. This explains why OM under Douglas fir with highest δ13C values in litterfall and roots exhibited the highest δ13C values throughout the soil profile, while they were lowest under oak.
In coincidence with the 13C enrichment with increasing soil depth, gradients of δ15N from the OL down to the 10–30 cm layer of mineral soil had developed in all forest stands during 35 years of afforestation (Fig. 2d). The depth distribution of SOM δ15N mainly results from an interplay of input signatures and losses that occur during decomposition processes (Craine et al. 2015). The accumulation of 15N-depleted plant litter on the soil surface determines the gradient from the significantly lower δ15N values of forest floor horizons to the 15N-enriched mineral soil. Thus, the highest δ15N values in the forest floor horizons under Douglas fir reflect the highest δ15N values of the litterfall and roots of all tree species that in turn were determined by the nitrate concentrations of Douglas fir soils (Zeller et al. 2019). The lowest forest floor δ15N values that were observed in the pine stand are in accordance with other studies revealing that conifer-dominated sites were 15N-depleted compared to deciduous species (Pardo et al. 2007). In contrast to the δ13C depth gradients, δ15N increased consistently throughout the soil profile following a curve that is typical for N-limited forest ecosystems dominated by EM fungi (Hobbie and Ouimette 2009). The clearly higher δ15N values under beech and Douglas fir compared to oak and pine are in accordance with observations made in a common garden experiment in Poland (Angst et al. 2019). With increasing depth and ongoing decomposition, SOM becomes preferentially 15N-enriched due to microbial activity coupled with an increasing proportion of 15N-enriched microbial derived compounds (Lerch et al. 2011). The individual SOM δ15N depth gradients of tree species converged in the upper two mineral soil horizons (0–5, 5–10 cm) and diverged again with increasing depth implying that SOM turnover differed under the influence of tree species. Additionally, tree species and their mycorrhizal symbionts, respectively, also contributes to the δ15N depth profiles by their N uptake from soil (Handley and Raven 1992; Callesen et al. 2013). The type of mycorrhizal association mainly drives the form of N acquisition of temperate tree species (Liese et al. 2017) and leads to differences in 15N enrichments in soil profiles between EM and arbuscular mycorrhizal fungi dominated systems (Hobbie and Högberg 2012). The here investigated tree species are all dominated by EM fungi with similar fungal biomasses (Wang and Qiu 2006; Awad et al. 2019) and thus, the N transfer from the soil to the host plant is presumably not significantly different between tree species. Anyhow, the turnover of SOM and the N uptake by plants are highly interrelated and therefore both mechanisms will have contributed to the δ15N depth profiles.
The negative relationship between δ15N and soil C:N ratio may result from increasing loss of 15N-depleted N in the form of nitrate leaching or denitrification as consequence of decreasing N retention (Marty et al. 2019). However, this option is likely subordinate because N-limited temperate forests are characterized by a largely closed internal N cycle, where N-losses are generally low due to a high competition for this growth-limiting resource (Rennenberg et al. 2009). Rather, the relationship of δ15N to the soil C:N is best explained by the increase in OM decomposition with increasing soil depth. The relationship between δ13C and soil C:N ratio, that is also negative, supports this assumption (Baisden et al. 2002). The well-known decline of the C:N ratio with increasing depth (Marín-Spiotta et al. 2014) is mostly attributed to OM decay because substrate that accumulates at the soil surface has significantly higher C:N ratios compared to decomposers and their products (Manzoni et al. 2010; Paul 2016). The slopes of the specific regression lines (Fig. 3a) were more driven by the enrichment of 15N with increasing depth than by the C:N ratio. Steeper slopes in beech and pine stands were associated with the significantly higher εsoil15N values of beech and pine compared to oak and Douglas fir. Nonetheless, the significant relationship between δ15N and the C:N ratio emphasizes the potential of 15N depth gradients as proxy for OM decay. Kramer et al. (2017) found that changes in organo-mineral associations can drive depth trends of C:N and δ15N more than the microbial decay. However, this effect was largely reduced, since forest stands with uniform mineral phase were investigated in this study. Furthermore, the O:C ratio that represents the state of chemical oxidation (Fan et al. 2018) of SOM was also significantly related to δ15N and δ13C throughout the soil profile. Oxidation is accompanied with microbial breakdown and depolymerization of plant residues followed by assimilation of C in microbial biomass as well as mineralization at the same time (Lehmann and Kleber 2015). Thus, with increasing depth the rise of the O:C ratio indicated the progressive oxidative degradation of OM and correlated with the enrichment of 15N and 13C in SOM. All this led to the assumption that the depth trends of SOM δ15N and δ13C resulted mainly from the decomposition of OM.
βδ13C values and εsoil15N and the contribution of litterfall and root inputs
In well-drained forest soils, like our study site ‘Sophienhöhe’, the linear regression function of δ13C and the logarithm of SOC with soil depth, termed as βδ13C value, is a suitable indicator of isotopic fractionation during decomposition (Brunn et al. 2014). Physical soil mixing processes that could also have a contribution to the isotopic fractionation with soil depth (Acton et al. 2013) can be excluded because during field surveys no earthworms or signs of significant bioturbation processes were found (Lorenz and Thiele-Bruhn 2019). However, steeper regression slopes, and more negative βδ13C values respectively, indicate higher rates of 13C enrichment through the soil depth profile and enhanced organic matter turnover (Garten 2006; Wang et al. 2018). Tree species had a significant effect on βδ13C values. The most negative βδ13C values and therefore the highest rates of SOM turnover were determined in beech forest stands, while reduced SOM turnover at coniferous sites was indicated by less negative βδ13C values. This is in accordance with the view that turnover rates, especially in the early-stage of decomposition, of deciduous species litter are generally higher compared to conifers (Augusto et al. 2015 and references in there). Long-term studies (> 10 years) suggest that there are also significant differences in the remaining masses after decomposition between tree species (Harmon et al. 2009; Prescott 2010). This can be addressed to significantly higher N contents and lower C:N ratios in the litterfall of beech and oak (Table S3), because it is well documented that these parameters correlate well with decomposition (Fernandez et al. 2003; Laganière et al. 2010; Vesterdal et al. 2012). Our findings are confirmed by other studies revealing that more negative βδ13C values were related to higher N contents and lower C:N ratios of litterfall (Garten et al. 2000; Garten 2006; Wang et al. 2015). The multiple linear regression analysis, which included different quantity and quality properties of the litter inputs, pointed out that the isotopic signatures of litterfall and root C:N account for nearly half of the variation (46%) in βδ13C values. This implies on the one hand that the initial isotopic composition of the aboveground litter plays a crucial role, for evaluating the 13C enrichment in soil depth profiles in the context of SOM turnover. Camino-Serrano et al. (2019) figured out that litter δ13C is the key to predict and model δ13C depth profiles. On the other hand, the C and N stoichiometry of root biomass seems to be of high importance for βδ13C values in forest soils. Belowground inputs are still less researched but knowledge is growing that these inputs have a significantly contribution to OM formation (Angst et al. 2018; Poirier et al. 2018). For example, Kramer et al. (2010) demonstrated that root-derived C is the major (> 60%) source of C for microbes in temperate deciduous forest soils, while also in boreal forest soils 50 to 70% of the stored C derives from roots and root-associated microorganisms (Clemmensen et al. 2013). However, 54% of the variation in βδ13C values cannot be explained with the here investigated properties of litterfall inputs and root material suggesting that SOM decomposition depends additionally on other factors.
The use of bulk SOM δ15N as proxy for turnover and stabilization of SOM bears an uncertainty due to factors like the variability of the initial abundance of 15N in litter inputs (Högberg 1997). Thus, we evaluated soil enrichment factors (εsoil15N) to level out the different isotopic signatures of litter inputs. Like the βδ13C values, εsoil15N values were significantly affected by tree species but the differences between tree species are not consistent for εsoil15N and βδ13C values. If mainly microbial turnover of SOM would drive both parameters in similar trajectories, this would result in a negative relationship between both indices. This applies for Douglas fir, oak and in particular for beech with oppositely high or low βδ13C and εsoil15N values, respectively (Fig. 4). However, soils under pine were characterized by highest βδ13C as well as high εsoil15N values. This indicates that in pine stands 13C and 15N enrichments with soil depth are decoupled from each other and not systematically interlinked. Nel et al. (2018) reported on a general link between δ13C and δ15N on global scale and with soil depth but they also mentioned that this link varied with local influences from biota, disturbance and environmental conditions. Multiple linear regression analysis revealed that litterfall C:N and δ15N as well as root O:C and δ13C account for 74% of the variability in εsoil15N. This highlights the importance of litterfall and root inputs for the 15N enrichment with soil depth. The general view that the higher decomposition rates of litter with high N content and low C:N ratio (Garten et al. 2000) are associated with both, high 15N and 13C enrichment does not match with the finding of high 15N enrichment in soils under pine. This must be caused by other processes. The high C:N ratio of the N-poor litterfall (Table S3) suggests that the N supply in the upper 30 cm of soil in the pine stands is limited. The significantly lowest root biomasses of the typical deep rooting tap root system (Burylo et al. 2011) combined with its low turnover rates (Yuan and Chen 2010) will also provide not much N. Under such N-limited conditions EM fungi are able to oxidize OM primarily as N source rather than as a source of metabolic C (Lindahl and Tunlid 2015). Thus, EM fungi in the pine stands can compete directly with decomposers for soil N resources and exacerbate the N-limitation of free-living decomposers (Averill 2016). Therefore, the soil microbial community could adapt their N-utilization strategy to an efficient re-use of organic N derived from their own bio- and necromass. The multiple recycling of these N sources will lead to an ongoing enrichment of 15N without higher rates of decomposition and could end up in the discrepancy between βδ13C and εsoil15N values. Furthermore, it has been reported that under conditions with low C availability, N-containing organic compounds can be primarily used as source of C and energy. Consequently, dissimilated 15N-depleted N will be exported and the microbial cell gets enriched relative to its source (Dijkstra et al. 2008). Consequently, both mechanisms, the effective recycling of microbial derived N under N-limited conditions as well as the dissimilation of 15N-enriched N, when the relative availability of C is low, could lead to high εsoil15N and low βδ13C values in pine stands.
Conclusion
The post-mining site “Sophienhöhe” represented a suitable site to characterize the influence of tree species on the natural abundance of 13C and 15N in soil depth gradients. As an advantage to many common garden experiments, an interference from old C sources originating from former land use was negligible. Additionally, 35 years after the afforestation were sufficient to generate tree species-specific depth gradients. Hence, evidence was provided that differences in isotopic signatures of SOM originated from the input of plant litter and its decomposition products.
The significantly different δ13C and δ15N values in the OM of forest floor and mineral soil reflected the signatures of the litter inputs (litterfall and roots) that were tree species specific. Along the soil profile, both isotopes were significantly related to the C:N and O:C ratio indicating that the enrichment of 13C and 15N with increasing soil depth is driven by processes that presumably can be assigned to microbial decomposition of OM. Consequently, when 13C and 15N of bulk SOM are used to evaluate decomposition and stabilization of OM, the isotopic signatures of litter inputs should be considered as well. Differences in βδ13C values indicated different turnover of SOM between tree species with higher decomposition rates in deciduous forest stands compared to conifers. The quality of litterfall and root inputs (N content, C:N, O:C ratio) as well as the initial isotopic signatures of litterfall contributed to the regulation of OM decomposition. Yet, 54% of the variance in βδ13C, and 26% in εsoil15N respectively, cannot be explained with the here investigated litterfall and root properties showing that SOM decomposition depends additionally on other—presumably microbial driven—factors. The correspondence of εsoil15N values with βδ13C values in three of the four investigated forest stands (Douglas fir, beech, oak) suggests that the 13C and 15N enrichment with increasing depth followed similar principles. However, the conditions under pine did not follow the systematic link between 13C and 15N enrichment. This is presumably due to specific N cycling mechanisms mediated by microorganisms that were adapted to conditions of limited N availability and the relatively low availability of C.
It is concluded that typical pattern of 13C and 15N enrichment with increasing soil depth are due to maturation and ongoing turnover of SOM. However, under the influence of tree species the enrichment of both isotopes did not follow similar trajectories in general because of microorganisms that can create specific utilization strategies depending on the litter quality. It was possible to obtain this finding by combining stable isotope analysis with the classical determination of stoichiometry ratios (C:N, O:C).
References
Acton P, Fox J, Campbell E, Rowe H, Wilkinson M (2013) Carbon isotopes for estimating soil decomposition and physical mixing in well-drained forest soils. J Geophys Res Biogeosci 118(4):1532–1545. https://doi.org/10.1002/2013JG002400
Andrianarisoa KS, Zeller B, Poly F, Siegenfuhr H, Bienaimé S, Ranger J, Dambrine E (2010) Control of nitrification by tree species in a common-garden experiment. Ecosystems 13:1171–1187. https://doi.org/10.1007/s10021-010-9390-x
Angst G, Messinger J, Greiner M, Häusler W, Hertel D, Kirfel K, Kögel-Knabner I, Leuschner C, Rethemeyer J, Mueller CW (2018) Soil organic carbon stocks in topsoil and subsoil controlled by parent material, carbon input in the rhizosphere, and microbial-derived compounds. Soil Biol Biochem 122:19–30. https://doi.org/10.1016/j.soilbio.2018.03.026
Angst G, Mueller KE, Eissenstat DM, Trumbore S, Freeman KH, Hobbie SE, Chorover J, Olekysn J, Reich PB, Mueller CW (2019) Soil organic carbon stability in forests: distinct effects of tree species identity and traits. Glob Change Biol 25:1529–1546. https://doi.org/10.1111/gcb.14548
Augusto L, De Schrijver A, Vesterdal L, Smolander A, Prescott C, Ranger J (2015) Influences of evergreen gymnosperm and deciduous angiosperm tree species on the functioning of temperate and boreal forests. Biol Rev 90:444–466. https://doi.org/10.1111/brv.12119
Averill C (2016) Slowed decomposition in ectomycorrhizal ecosystems is independent of plant chemistry. Soil Biol Biochem 102:52–54. https://doi.org/10.1016/j.soilbio.2016.08.003
Awad A, Majcherczyk A, Schall P, Schröter K, Schöning I, Schrumpf M, Ehbrecht M, Boch S, Kahl T, Bauhus J, Seidel D, Ammer C, Fischer M, Kües U, Pena R (2019) Ectomycorrhizal and saprotrophic soil fungal biomass are driven by different factors and vary among broadleaf and coniferous temperate forests. Soil Biol Biochem 131:9–18. https://doi.org/10.1016/j.soilbio.2018.12.014
Badeck FW, Tcherkez G, Nogués S, Piel C, Gashghaie J (2005) Post-photosynthetic fractionation of stable carbon isotopes between plant organs—a widespread phenomenon. Rapid Commun Mass Spectrom 19:1381–1391. https://doi.org/10.1002/rcm.1912
Baisden WT, Amundson R, Cook AC, Brenner DL (2002) Turnover and storage of C and N in five density fractions from California annual grassland surface soils. Glob Biogeochem Cycles 16:64-1–64-16. https://doi.org/10.1029/2001GB001822
Balesdent J, Mariotti A, Guillet B (1987) Natural 13C abundance as a tracer for studies of soil organic matter dynamics. Soil Biol Biochem 19:25–30. https://doi.org/10.1016/0038-0717(87)90120-9
Balesdent J, Basile-Doelsch I, Chadoeuf J, Cornu S, Derrien D, Fekiacova Z, Hatté C (2018) Atmosphere–soil carbon transfer as a function of soil depth. Nature 559:599–602. https://doi.org/10.1038/s41586-018-0328-3
Berg B (2008) Litter decomposition and organic matter turnover in northern forest soils. For Ecol Manag 133:13–22. https://doi.org/10.1016/S0378-1127(99)00294-7
Berg B, McGlaugherty C (2014) Plant litter. Decomposition, humus formation, carbon sequestration, 3rd edn. Springer, Berlin
Beyer L, Deslis K, Vogt B (1998) Estimation of soil organic matter composition according to a simple thermoanalytical approach. Commun Soil Sci Plant Anal 29:1277–1297. https://doi.org/10.1080/00103629809370026
Billings SA, Richter D (2006) Changes in stable isotopic signatures of soil nitrogen and carbon during 40 years of forest development. Oecologia 148:325–333. https://doi.org/10.1007/s00442-006-0366-7
Bird MI, Kracht O, Derrien D, Zhou Y (2003) The effect of soil texture and roots on the stable isotope composition of soil organic matter. Aust J Soil Res 41:77–94. https://doi.org/10.1071/SR02044
Boström B, Comstedt D, Ekblad A (2007) Isotope fractionation and 13C enrichment in soil profiles during the decomposition of soil organic matter. Oecologia 153:89–98. https://doi.org/10.1007/s00442-007-0700-8
Bowling DR, Pataki DE, Randerson JT (2008) Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes. New Phytol 178:24–40. https://doi.org/10.1111/j.1469-8137.2007.02342.x
Brooks JR, Flanagan LB, Buchmann N, Ehleringer JR (1997) Carbon isotope composition of boreal plants: functional grouping of life forms. Oecologia 110:301–311. https://doi.org/10.1007/s004420050163
Brüggemann N, Gessler A, Kayler Z, Keel Z, Badeck F, Barthel M, Boeckx P, Buchmann N, Brugnoli E, Esperschütz J, Gavrichkova O, Ghashghaie J, Gomez-Casanovas N, Keitel C, Knowhl A, Kuptz D, Palacio S, Salmon Y, Uchida Y, Bahn M (2011) Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: a review. Biogeosciences 8:3457–3489. https://doi.org/10.5194/bg-8-3457-2011
Brunn M, Spielvogel S, Sauer T, Oelmann Y (2014) Temperature and precipitation effects on δ13C depth profiles in SOM under temperate beech forests. Geoderma 235–236:146–153. https://doi.org/10.1016/j.geoderma.2014.07.007
Brunn M, Brodbeck S, Oelmann Y (2017) Three decades following afforestation are sufficient to yield δ13C depth profiles. J Plant Nutr Soil Sc 180:643–647. https://doi.org/10.1002/jpln.201700015
Burylo M, Hudek C, Rey F (2011) Soil reinforcement by the roots of six dominant species on eroded mountainous marly slopes (Southern Alps, France). CATENA 84:70–78. https://doi.org/10.1016/j.catena.2010.09.007
Callesen I, Nilsson LO, Schmidt IK, Vesterdal L, Ambus P, Christiansen JR, Högberg P, Gundersen P (2013) The natural abundance of 15N in litter and soil profiles under six temperate tree species: N cycling depends on tree species traits and site fertility. Plant Soil 368:375–392. https://doi.org/10.1007/s11104-012-1515-x
Camino-Serrano M, Tifafi M, Balesdent J, Hatté C, Peñuelas J, Cornu S, Guenet B (2019) Including stable carbon isotopes to evaluate the dynamics of soil carbon in the land-surface model ORCHIDEE. J Adv Model Earth Syst 11:3650–3669. https://doi.org/10.1029/2018MS001392
Cernusak LA, Tcherkez G, Keitel C, Cornwell WK, Santiago LS, Knohl A, Barbour MM, Williams DG, Reich PB, Ellsworth DS, Dawson TE, Griffiths HG, Farquhar GD, Wright IJ (2009) Why are non-photosynthetic tissues generally 13C enriched compared with leaves in C3 plants? Review and synthesis of current hypotheses. Funct Plant Biol 36:199–213. https://doi.org/10.1071/FP08216
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlid J, Finlay RD, Wardle DA, Lindahl BD (2013) Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339:1615–1618. https://doi.org/10.1126/science.1231923
Cools N, Vesterdal L, De Vos B, Vanguelova E, Hansen K (2014) Tree species is the major factor explaining C:N ratios in European forest soils. For Ecol Manag 311:3–16. https://doi.org/10.1016/j.foreco.2013.06.047
Craine JM, Elmore AJ, Aidar MPM, Bustamante M, Dawson TE, Hobbie EA, Kahmen A, Mack MC, McLauchlan KK, Michelsen A, Nardoto GB, Pardo LH, Peñuela J, Reich PB, Schuur G, Stock WD, Templer PH, Virginia RA, Welker JM, Wright IJ (2009) Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol 183:980–992. https://doi.org/10.1111/j.1469-8137.2009.02917.x
Craine JM, Brookshire ENJ, Cramer MD, Hasselquist NJ, Koba K, Marin-Spiotta E, Wang L (2015) Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 396:1–26. https://doi.org/10.1007/s11104-015-2542-1
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33:507–559. https://doi.org/10.1146/annurev.ecolsys.33.020602.095451
Dijkstra P, Ishizu A, Doucett R, Hart SC, Schwartz E, Menyailo OV, Hungate BA (2006) 13C and 15N natural abundance of the soil microbial biomass. Soil Biol Biochem 38:3257–3266. https://doi.org/10.1016/j.soilbio.2006.04.005
Dijkstra P, LaViolette CM, Coyle JS, Doucett RR, Schwartz E, Hart SC, Hungate BA (2008) 15N enrichment as an integrator of the effects of C and N on microbial metabolism and ecosystem function. Ecol Lett 11:389–397. https://doi.org/10.1111/j.1461-0248.2008.01154.x
Dümig A, Rumpel C, Dignac MF, Kögel-Knabner I (2013) The role of lignin for the δ13C signature in C4 grassland and C3 forest soils. Soil Biol Biochem 57:1–13. https://doi.org/10.1016/j.soilbio.2012.06.018
Ehleringer JR, Buchmann N, Flanagan LB (2000) Carbon isotope ratios in belowground carbon cycle processes. Ecol Appl 10(2):412–422
Emmet BA, Kjønaas OJ, Gundersen P, Koopmans C, Tietema A, Sleep D (1998) Natural abundance of 15N in forests across a nitrogen deposition gradient. For Ecol Manag 101:9–18. https://doi.org/10.1016/S0378-1127(97)00121-7
Fan F, Henriksen CB, Porter J (2018) Relationship between stoichiometry and ecosystem services: a case study of organic farming systems. Ecol Indic 85:400–408. https://doi.org/10.1016/j.ecolind.2017.10.063
Fernandez I, Mahieu N, Cadisch G (2003) Carbon isotopic fractionation during decomposition of plant materials of different quality. Glob Biogeochem Cycles 17:1075. https://doi.org/10.1029/2001GB001834
Francey RJ, Allison CE, Etheridge DM, Trudinger CM, Enting IG, Leuenberger M, Langenfelds RL, Michel E, Steele LP (1999) A 1000-year high precision record of δ13C in atmospheric CO2. Tellus 51B:170–193. https://doi.org/10.1034/j.1600-0889.1999.t01-1-00005.x
Frouz J, Pižl V, Cienciala E, Kalčík J (2009) Carbon storage in post-mining forest soil, the role of tree biomass and soil bioturbation. Biogeochemistry 94:111–121. https://doi.org/10.1007/s10533-009-9313-0
Garten CT (2006) Relationships among forest soil C isotopic composition, partitioning, and turnover times. Can J For Res 36:2157–2167. https://doi.org/10.1139/x06-115
Garten CT, Cooper LW, Post M, Hanson PJ (2000) Climate controls on forest soil C isotope ratios in the southern Appalachian Mountains. Ecology 81(4):1108–1119
Gurmesa GA, Schmidt IK, Gundersen P, Vesterdal L (2013) Soil carbon accumulation and nitrogen retention traits of four tree species grown in common gardens. For Ecol Manag 309:47–57. https://doi.org/10.1016/j.foreco.2013.02.015
Handley LL, Raven JA (1992) The use of natural abundance of nitrogen isotopes in plant physiology and ecology. Plant, Cell Environ 15:965–985. https://doi.org/10.1111/j.1365-3040.1992.tb01650.x
Harmon ME, Silver WL, Fasth B, Chen H, Burke IC, Parton WJ, Hart SC, Currie WS, LIDET (2009) Long-term patterns of mass loss during the decomposition of leaf and fine root litter: an intersite comparison. Glob Change Biol 15:1320–1338. https://doi.org/10.1111/j.1365-2486.2008.01837.x
Hobbie EA, Colpaert JV (2003) Nitrogen availability and colonization by mycorrhizal fungi correlate with nitrogen isotope patterns in plants. New Phytol 157:115–126. https://doi.org/10.1046/j.1469-8137.2003.00657.x
Hobbie EA, Högberg P (2012) Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol 196(2):367–382. https://doi.org/10.1111/j.1469-8137.2012.04300.x
Hobbie EA, Ouimette AP (2009) Controls of nitrogen isotope patterns in soil profiles. Biogeochemistry 95:355–371. https://doi.org/10.1007/s10533-009-9328-6
Hobbie EA, Werner RA (2004) Intramolecular, compound-specific, and bulk carbon isotope patterns in C3 and C4 plants: a review and synthesis. New Phytol 161:371–385. https://doi.org/10.1046/j.1469-8137.2004.00970.x
Högberg P (1997) 15N natural abundance in soil-plant systems. New Phytol 137:179–203. https://doi.org/10.1046/j.1469-8137.1997.00808.x
Högberg P, Högbom L, Schinkel H, Högberg M, Johannisson C, Wallmark H (1996) 15N abundance of surface soils, roots and mycorrhizas in profiles of European forest soils. Oecologia 108:207–214. https://doi.org/10.1007/BF00334643
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33. https://doi.org/10.1007/s11104-009-9925-0
Kramer C, Trumbore S, Fröberg M, Cisneros Dozal LM, Zhang D, Xu X, Santos GM, Hanson PJ (2010) Recent (< 4 year old) leaf litter is not a major source of microbial carbon in a temperate forest mineral soil. Soil Biol Biochem 42:1028–1037. https://doi.org/10.1016/j.soilbio.2010.02.021
Kramer MG, Lajtha K, Aufdenkampe AK (2017) Depth trends of soil organic matter C:N and 15 N natural abundance controlled by association with minerals. Biogeochemistry 136:237–248. https://doi.org/10.1007/s10533-017-0378-x
Laganière J, Paré D, Bradley RL (2010) How does a tree species influence litter decomposition? Separating the relative contribution of litter quality, litter mixing, and forest floor conditions. Can J For Res 40:465–475. https://doi.org/10.1139/X09-208
Lehmann J, Kleber M (2015) The contentious nature of soil organic matter. Nature 528:60–68. https://doi.org/10.1038/nature16069
Lerch TZ, Nunan N, Dignac MF, Chenu C, Mariotti A (2011) Variations in microbial isotopic fractionation during soil organic matter decomposition. Biogeochemistry 106(1):5–21. https://doi.org/10.1007/s10533-010-9432-7
Liese R, Lübbe T, Albers NW, Meier IC (2017) The mycorrhizal type governs root exudation and nitrogen uptake of temperate tree species. Tree Physiol 38:83–95. https://doi.org/10.1093/treephys/tpx131
Lindahl BD, Tunlid A (2015) Ectomycorrhizal fungi—potential organic matter decomposers, yet not saprotrophs. New Phytol 205:1443–1447. https://doi.org/10.1111/nph.13201
Lorenz M, Thiele-Bruhn S (2019) Tree species affect soil organic matter stocks and stoichiometry in interaction with soil microbiota. Geoderma 353:35–46. https://doi.org/10.1016/j.geoderma.2019.06.021
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80:89–106. https://doi.org/10.1890/09-0179.1
Marín-Spiotta E, Gruley KE, Crawford J, Atkinson EE, Miesel JR, Greene S, Cardona-Correa C, Spencer GM (2014) Paradigm shifts in soil organic matter research affect interpretations of aquatic carbon cycling: transcending disciplinary and ecosystem boundaries. Biogeochemistry 117:279–297. https://doi.org/10.1007/s10533-013-9949-7
Marty C, Houle D, Gagnon C (2015) Effect of the relative abundance of conifers versus hardwoods on soil δ13C enrichment with soil depth in Eastern Canadian forests. Ecosystems 18:629–642. https://doi.org/10.1007/s10021-015-9852-2
Marty C, Houle D, Courchesne F, Gagnon (2019) Soil C:N ratio is the main driver of soil δ15 N in cold and N-limited eastern Canadian forests. CATENA 172:285–294. https://doi.org/10.1016/j.catena.2018.08.029
Mueller KE, Eissenstat DM, Hobbie SE, Oleksyn J, Jagodzinski AM, Reich PB, Chadwick OA, Chorover J (2012) Tree species effects on coupled cycles of carbon, nitrogen, and acidity in mineral soils at a common garden experiment. Biogeochemistry 111:601–614. https://doi.org/10.1007/s10533-011-9695-7
Mueller KE, Hobbie SE, Chorover J, Reich PB, Eisenhauer N, Castellano MJ, Chadwick OA, Dobies T, Hale CM, Jagodziński AM, Kałucka I, Kieliszewska-Rokicka B, Modrzyński J, Rożen A, Skorupski M, Sobczyk Ł, Stasińska M, Trocha LK, Weiner J, Wierzbicka A, Oleksyn J (2015) Effects of litter traits, soil biota, and soil chemistry on soil carbon stocks at a common garden with 14 tree species. Biogeochemistry 123:313–327. https://doi.org/10.1007/s10533-015-0083-6
Nadelhoffer KJ, Fry B (1988) Controls on natural nitrogen-15 and carbon-13 abundances in forest soil organic matter. Soil Sci Soc Am J 52(6):1633–1640. https://doi.org/10.2136/sssaj1988.03615995005200060024x
Nel JA, Craine JM, Cramer MD (2018) Correspondence between δ13C and δ15N in soils suggests coordinated fractionation processes for soil C and N. Plant Soil 423(1–2):257–271. https://doi.org/10.1007/s11104-017-3500-x
Osono T, Takeda H, Azuma J (2008) Carbon isotope dynamics during leaf litter decomposition with reference to lignin fractions. Ecol Res 23:51–55. https://doi.org/10.1007/s11284-007-0336-5
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackso RB, Pacala SW, McGuire AD, Piao AR, Rautiainen A, Sitch S, Hayes (2011) A large and persistent carbon sink in the world’s forests. Science 333:988. https://doi.org/10.1126/science.1201609
Pardo LH, Hemond HF, Montoya JP, Pett-Ridge J (2007) Natural abundance 15N in soil and litter across a nitrate-output gradient in New Hampshire. For Ecol Manag 251:217–230. https://doi.org/10.1016/j.foreco.2007.06.047
Pardo LH, Semaoune P, Schaberg PG, Eagar C, Sebilo M (2013) Patterns in δ15N in roots, stems, and leaves of sugar maple and American beech seedlings, saplings, and mature trees. Biogeochemistry 112:275–291. https://doi.org/10.1007/s10533-012-9724-1
Paul EA (2016) The nature and dynamics of soil organic matter: plant inputs, microbial transformations, and organic matter stabilization. Soil Biol Biochem 98:109–126. https://doi.org/10.1016/j.soilbio.2016.04.001
Poirier V, Roumet C, Munson AD (2018) The root of the matter: linking root traits and soil organic matter stabilization processes. Soil Biol Biochem 120:246–259. https://doi.org/10.1016/j.soilbio.2018.02.016
Pörtl K, Zechmeister-Boltenstern S, Wanek W, Ambus P, Berger TW (2007) Natural 15N abundance of soil N pools and N2O reflect the nitrogen dynamics of forest soils. Plant Soil 295:79–94. https://doi.org/10.1007/s11104-007-9264-y
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149. https://doi.org/10.1007/s10533-010-9439-0
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reich PB, Oleksyn J, Modrzynski J, Mrozinski P, Hobbie SE, Eissenstat DM, Chorover J, Chadwick OA, Hale CM, Tjoelker (2005) Linking litter calcium, earthworms and soil properties: a common garden test with 14 tree species. Ecol Lett 8:811–818. https://doi.org/10.1111/j.1461-0248.2005.00779.x
Rennenberg H, Dannenmann M, Gessler A, Kreuzwieser J, Simon J, Papen H (2009) Nitrogen balance in forest soils nutritional limitation of plants under climate change stresses. Plant Biol 11:24–33. https://doi.org/10.1111/j.1438-8677.2009.00241.x
Schrumpf M, Kaiser K, Guggenberger G, Persson T, Kögel-Knabner I, Schulze ED (2013) Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals. Biogeosciences 10:1675–1691. https://doi.org/10.5194/bg-10-1675-2013
Schwartz E, Blazewicz S, Doucett R, Hungate BA, Hart SC, Dijkstra P (2007) Natural abundance δ15N and δ13C of DNA extracted from soil. Soil Biol Biochem 39:3101–3107. https://doi.org/10.1016/j.soilbio.2007.07.004
Silfer JA, Engel MH, Macko SA (1992) Kinetic fractionation of stable carbon and nitrogen isotopes during peptide bond hydrolysis: experimental evidence and geochemical implications. Chem Geol: Isotope Geosci Sect 101(3–4):211–221. https://doi.org/10.1016/0009-2541(92)90003-N
Stevenson FJ (1994) Humus chemistry—genesis, composition, reactions, 2nd edn. Wiley, New York
Suárez-Abelenda M, Ahmad R, Camps-Arbestain M, Herath SHMSK (2015) Changes in the chemical composition of soil organic matter over time in the presence and absence of living roots: a pyrolysis GC/MS study. Plant Soil 391:161–177. https://doi.org/10.1007/s11104-015-2423-7
Templer PH, Arthur MA, Lovett GM, Weathers KC (2007) Plant and soil natural abundance δ15N: indicators of relative rates of nitrogen cycling in temperate forest ecosystems. Oecologia 153:399–406. https://doi.org/10.1007/s00442-007-0746-7
Trumbore SE (2009) Radiocarbon and soil carbon dynamics. Annu Rev Earth Planet Sci 37:47–66. https://doi.org/10.1146/annurev.earth.36.031207.124300
Ukonmaanaho L, Pitman R, Bastrup-Birk A, Breda N, Rautio P (2016) Part XIII: Sampling and Analysis of Litterfall. In: UNECE ICP Forests Programme Co-ordinating Centre (ed) Manual on methods and criteria for harmonized sampling, assessment, monitoring and analysis of the effects of air pollution on forests. Thünen Institute for Forests Ecosystems, Eberswalde, Germany, 14 p. + Annex. http://www.icp-forests.org/manual.htm
Vallano DM, Sparks JP (2013) Foliar δ15N is affected by foliar nitrogen uptake, soil nitrogen, and mycorrhizae along a nitrogen deposition gradient. Oecologia 172:47–58. https://doi.org/10.1007/s00442-012-2489-3
Vesterdal L, Schmidt IK, Callesen I, Nilsson LO, Gundersen P (2008) Carbon and nitrogen in forest floor and mineral soil under six common European tree species. For Ecol Manag 225:35–48. https://doi.org/10.1016/j.foreco.2007.08.015
Vesterdal L, Elberling B, Christiansen JR, Callesen I, Schmidt IK (2012) Soil respiration and rates of soil carbon turnover differ among six common European tree species. For Ecol Manag 264:185–196. https://doi.org/10.1016/j.foreco.2011.10.009
Vesterdal L, Clarke N, Sigurdsson BD, Gundersen P (2013) Do tree species influence soil carbon stocks in temperate and boreal forests? For Ecol Manag 309:4–18. https://doi.org/10.1016/j.foreco.2013.01.017
von Lützow M, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H (2007) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Eur J Soil Sci 57:426–445. https://doi.org/10.1111/j.1365-2389.2006.00809.x
Wallander H, Mörth CM, Giesler R (2009) Increasing abundance of soil fungi is a driver for 15N enrichment in soil profiles along a chronosequence undergoing isostatic rebound in northern Sweden. Oecologia 160:87–96. https://doi.org/10.1007/s00442-008-1270-0
Wang B, Qiu YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363. https://doi.org/10.1007/s00572-005-0033-6
Wang G, Jia Y, Li W (2015) Effects of environmental and biotic factors on carbon isotopic fractionation during decomposition of soil organic matter. Sci Rep 5:11043. https://doi.org/10.1038/srep11043
Wang C, Houlton BZ, Liu D, Hou J, Cheng W, Bai E (2018) Stable isotopic constraints on global soil organic carbon turnover. Biogeosciences 15:987–995. https://doi.org/10.5194/bg-15-987-2018
Werth M, Kuzyakov Y (2010) 13C fractionation at the root–microorganisms–soil interface: a review and outlook for partitioning studies. Soil Biol Biochem 42:1372–1384. https://doi.org/10.1016/j.soilbio.2010.04.009
Wilske B, Eccard JA, Zistl-Schlingmann M, Hohmann M, Methler A, Herde A, Liesenjohann T, Dannenmann M, Butterbach-Bahl K, Breuer L (2015) Effects of short term bioturbation by common voles on biogeochemical soil variables. PLoS ONE 10(5):e0126011. https://doi.org/10.1371/journal.pone.0126011
Wynn JG, Harden JW, Fries TL (2006) Stable carbon isotope depth profiles and soil organic carbon dynamics in the lower Mississippi Basin. Geoderma 131:89–109. https://doi.org/10.1016/j.geoderma.2005.03.005
Yuan ZY, Chen HYH (2010) Fine root biomass, production, turnover rates, and nutrient contents in boreal forest ecosystems in relation to species, climate, fertility, and stand age: literature review and meta-analyses. Crit Rev Plant Sci 29:204–221. https://doi.org/10.1080/07352689.2010.483579
Zanella A, Ponge J-F, Jabiol B, Sartori G, Kolb E, Le Bayon R-C, Gobat J-M, Aubert M, De Waal R, Van Delft B, Vacca A, Serra G, Chersich S, Andreetta A, Kõlli R, Brun JJ, Cool N, Englisch M, Hager H, Katzensteiner K, Brêthes A, De Nicola C, Testi A, Bernier N, Graefe U, Wolf U, Juilleret J, Garlato A, Obber S, Galvan P, Zampedri R, Frizzera L, Tomasi M, Banas D, Bureau F, Tatti D, Salmon S, Menardi R, Fontanella F, Carraro V, Pizzeghello D, Concheri G, Squartini A, Cattaneo D, Scattolin L, Nardi S, Nicolini G, Viola F (2018) Humusica 1, article 5: terrestrial humus systems and forms—keys of classification of humus systems and forms. Appl Soil Ecol 122:75–86. https://doi.org/10.1016/j.apsoil.2017.06.012
Zechmeister-Boltenstern S, Keiblinger KM, Mooshammer M, Peñuelas J, Richter A, Sardans J, Wanek W (2015) The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol Monogr 85:133–155. https://doi.org/10.1890/14-0777.1
Zeller B, Legout A, Bienaimé S, Gratia B, Santenoise P, Bonnaud P, Ranger J (2019) Douglas fir stimulates nitrification in French forest soils. Sci Rep 9:10687. https://doi.org/10.1038/s41598-019-47042-6
Acknowledgements
The authors thank the colleagues of the Soil Science Department of Trier University, P. Ziegler and M. Ortner, and the students, K. Becker, L. von Drathen, S. Stein and L. Schneider, for assistance during laboratory and field work. We also thank the certified facility in Functional Ecology (PTEF OC 081) from UMR 1137 EEF and UR 1138 BEF in the research centre INRAE Grand-Est–Nancy, and in particular S. Moutama, J. Ph. Gallais for sample preparation and weighting and C. Hossann, for performing/supervising isotopic analyses. Many thanks to Oliver Brendel (INRA, Champenoux, France) for suggestions in the data interpretation. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The UR BEF is supported by the French National Research Agency through the Cluster of Excellence ARBRE (ANR-11-LABX-0002-01). Additional support was provided by the mobile lab (M-POETE) of ANAEE-France. ANAEE-France is an infrastructure from the French Investment for the Future (Investissements d’Avenir) program, overseen by the French National Research Agency (ANR-11-INBS-0001).
Funding
Open Access funding enabled and organized by Projekt DEAL..
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Edith Bai.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lorenz, M., Derrien, D., Zeller, B. et al. The linkage of 13C and 15N soil depth gradients with C:N and O:C stoichiometry reveals tree species effects on organic matter turnover in soil. Biogeochemistry 151, 203–220 (2020). https://doi.org/10.1007/s10533-020-00721-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00721-3