Abstract
Soil enzymes are catalysts of organic matter depolymerisation, which is of critical importance for ecosystem carbon (C) cycling. Better understanding of the sensitivity of enzymes to temperature will enable improved predictions of climate change impacts on soil C stocks. These impacts may be especially large in tropical montane forests, which contain large amounts of soil C. We determined the temperature sensitivity (Q 10) of a range of hydrolytic and oxidative enzymes involved in organic matter cycling from soils along a 1900 m elevation gradient (a 10 °C mean annual temperature gradient) of tropical montane forest in the Peruvian Andes. We investigated whether the activity (V max) of selected enzymes: (i) exhibited a Q 10 that varied with elevation and/or soil properties; and (ii) varied among enzymes and according to the complexity of the target substrate for C-degrading enzymes. The Q 10 of V max for β-glucosidase and β-xylanase increased with increasing elevation and declining mean annual temperature. For all other enzymes, including cellobiohydrolase, N-acetyl β-glucosaminidase and phosphomonoesterase, the Q 10 of V max did not vary linearly with elevation. Hydrolytic enzymes that degrade more complex C compounds had a greater Q 10 of V max, but this pattern did not apply to oxidative enzymes because phenol oxidase had the lowest Q 10 value of all enzymes studied here. Our findings suggest that regional differences in the temperature sensitivities of different enzyme classes may influence the terrestrial C cycle under future climate warming.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical forest soils contain 30 % of global soil carbon (C) (Jobbagy and Jackson 2000), a large portion of which is contained in montane forest soils due to low temperatures and slow rates of decomposition (Moser et al. 2011; Zimmermann et al. 2010c). Microbial degradation of soil organic matter is one of the largest sources of atmospheric CO2 emissions, and is predicted to increase in the future as soil biochemical reaction rates accelerate with increasing global temperature (Davidson and Janssens 2006; Knorr et al. 2005). This is of particular concern for tropical montane forests, which contain large soil C stocks and are predicted to warm considerably in the coming decades. For example, the recent increase in temperature in the tropical Andes has exceeded the global average and suggests a warming by 3–5 °C this century as a result of anthropogenic climate change (Vuille and Bradley 2000). As a result, there is an urgent need to understand the thermal stability of soil C stores in tropical montane forests.
The intrinsic temperature sensitivity of soil organic matter decomposition remains contentious, because it can be obscured by various environmental constraints (Davidson and Janssens 2006). The rate limiting step in the depolymerisation of organic matter, at least while it remains physically accessible to heterotrophic micro-organisms, is the activity of extracellular enzymes (Burns and Staunton 2013). However, we lack basic information on how the influence of soil enzymes on soil organic matter cycling will change under future climatic change (Bradford 2013; Conant et al. 2011). For example, higher temperatures have been predicted to reduce the C-use efficiency of decomposers, thus reducing substrate degradation and increasing soil C stocks (Allison et al. 2010). However, the model this prediction is based on assumes a uniform response of different enzymes and enzyme classes to temperature following Arrhenius kinetics, missing potential variation among enzyme classes. For example, enzymes that degrade more complex C compounds may be more sensitive to temperature than those that degrade simple compounds (Davidson and Janssens 2006; Fierer et al. 2006). Further complexity in the temperature response of enzymes is indicated by studies showing seasonal and geographical variation in the temperature sensitivity of different enzymes (German et al. 2012; Wallenstein et al. 2009). Although important determinants of the overall temperature sensitivity of soil C, the temperature response of enzyme catalytic rates remain poorly characterised in models (Wieder et al. 2013).
Soil enzymes are synthesized by microorganisms or roots to degrade specific organic compounds by hydrolysis (hydrolytic enzymes) or oxidation (oxidative enzymes) (Burns and Staunton 2013). Soil organic matter cycling in a warmer climate would be strongly affected if different enzyme classes exhibit different temperature responses, or if enzyme properties are ‘acclimated’ to different environments (Wallenstein et al. 2011). The potential for large effects from temperature-acclimated enzymes has been illustrated by studies showing that enzymes from colder environments tend to be more responsive to rising temperature than enzymes from warmer environments (Dong and Somero 2009; Koch et al. 2007; Somero 2004). This ‘cold-adaptation’ is believed to be a consequence of differences in protein structure and enzyme conformation, with cold-adapted enzymes more sensitive to temperature increases and more vulnerable to denaturing at higher temperatures (Hochachka and Somero 2002). Enzyme temperature sensitivities may also be a consequence of temperature effects on substrate availability, desorption reactions and microbial C-use efficiency (Wallenstein et al. 2011), and their temperature response may be obscured by physical obstruction between enzymes and the substrates they degrade (Zimmermann et al. 2012).
Given the lack of understanding of how climate warming will affect soil organic matter cycling in tropical montane forests, we investigated the temperature response of a range of enzymes involved in organic matter cycling (Table 1) along a tropical montane forest elevation gradient of 1900 m, where mean annual temperature (MAT) ranges from 7 °C at the highest site to 17 °C at the lowest site. This gradient provides valuable insight into the long-term temperature adaptation of enzymes because the study sites along this gradient do not experience significant seasonality in temperature (Rapp and Silman 2012) and their soils remain moist year-round (Zimmermann et al. 2010a); as a result, the main abiotic influence on enzyme activity is the elevation-related temperature difference. Previous studies along this gradient have shown that the temperature sensitivity of soil heterotrophic respiration increases with increased elevation (Zimmermann et al. 2009), which may be related to differences in resource availability, enzyme activity and the soil microbial community composition (Whitaker et al. 2014). Here we build upon these studies and ask whether differences in soil organic matter cycling are directly affected by the temperature response of enzymatic reactions.
We hypothesised: (1) greater temperature sensitivity (Q 10) of enzymes from higher elevations (‘cold adapted’), and (2) greater intrinsic temperature sensitivity of enzymes that degrade complex organic molecules (e.g. lignin) relative to simple organic molecules (e.g. glucose). Here we explicitly tested whether different enzymatic reactions have temperature sensitivities relating to differences in mean annual temperature, and whether differences in enzyme activities relate to substrate availability is addressed elsewhere (Nottingham et al. 2015). We tested these hypotheses by measuring the temperature sensitivity (Q 10) of activity (V max) for a range of soil enzymes involved in the degradation of C (β-glucosidase, β-xylanase, cellobiohydrolase, phenol oxidase), nitrogen (N) (N-acetyl β-glucosaminidase) and phosphorus (P) (phosphomonoesterase) taken from a 1900 m elevation gradient in the Peruvian Andes.
Materials and methods
Study sites
The ten study sites are situated along an elevation gradient on the eastern flank of the Peruvian Andes (ranging in elevation from 1500 to 3400 m asl (above sea level) across 35 km distance) and are under continuous forest (ranging from lower to upper montane tropical forest). Mean annual temperature decreases with increasing elevation from 17.4 to 7.7 °C. Mean annual precipitation does not vary consistently with elevation (ranging from 1560 to 5302 mm yr−1 with some inter-annual variability indicated by a range of reported values; the most recent indicate a peak at mid-elevation; Malhi, unpublished data). Evidence to date indicates that plants and soils at all sites are rarely moisture limited over the seasonal cycle (van de Weg et al. 2009, 2014; Zimmermann et al. 2010b).
The sites are situated predominantly on Paleozoic (~450 Ma) meta-sedimentary mudstones with plutonic intrusions (granite) underlying the sites between 1500 and 2020 m asl (Carlotto et al. 1996; Clark et al. 2013). In the Soil Taxonomy classification system the soils are Inceptisols throughout the gradient (in the FAO World Reference Base soils at higher elevation are Umbrisols and at lower elevation are Cambisols). The soils at higher elevations are shallower to bedrock but have deeper organic horizons (Table 2). Further information on the sites and descriptions of the soils (Quesada et al. 2010), climate (Rapp and Silman 2012), above-ground productivity and floristic composition (Asner et al. 2014; Feeley et al. 2011; Girardin et al. 2010) are reported elsewhere.
Soil sampling and analyses
For determination of enzyme activity (V max) and all parameters in Table 2, organic soil was sampled in December 2010 from five sub-plots within a 1 ha plot at each of the 10 study sites. Because December falls in the early wet season for all study sites, soil microbes were not water limited during sample collection (Rapp and Silman 2012). For each sub-plot, soil was removed from a 40 × 40 cm area to 10 cm depth from the organic horizon. Soils were sealed in plastic bags and stored at 4 °C for less than 4 weeks until analysis. The storage period was considered acceptable because all soils were stored for the same amount of time and there are minimal changes in hydrolytic enzyme activities in lowland tropical soils during a month of storage at 4 °C (Turner and Romero 2010).
Total C and N were determined for dried, ground soil samples using a TruSpec CN Elemental Determinator (LECO, USA). Total P was determined by ignition (550 °C, 1 h) followed by extraction in 1 M H2SO4, with phosphate detection in neutralised extracts at 880 nm by automated molybdate colorimetry using a Lachat Quikchem 8500 (Hach Ltd, Loveland, CO, USA). Soil pH was determined in deionized H2O. Gravimetric moisture content, bulk density (dried for 24 h at 105 °C) and water holding capacity (the amount of water remaining in the soil 12 h after being saturated) were calculated for composite soil samples from each site.
Soil enzymes
The temperature sensitivity of enzyme activity (Q 10 of V max) was determined for six enzymes involved in C, N and P cycling at all 10 study sites. We also determined the temperature sensitivity of half saturation constants (Q 10 of Km), which is another important parameter determining rates of enzymatic SOM degradation. However, the determined Michaelis Menten models gave Km values with very high variance, suggestive of confounding temperature-interactions with substrate availability in different soils or of multiple-phase kinetics—the presence of different enzyme pools with different Km values, therefore we elected to focus only on V max responses in this study. Enzyme activity was measured using microplate fluorimetric assays with methylumbelliferone (MU)-linked substrates (Nottingham et al. 2011; Turner and Romero 2010): β-glucosidase (acts on β -bonds in glucose), cellobiohydrolase (acts on cellulose), N-acetyl β-glucosaminidase (acts on N-glycosidic bonds), phosphomonoesterase (acts on monoester-linked simple organic phosphates) and β-xylanase (acts on hemicellulose). Phenol oxidase (acts on phenolic compounds) was measured using 5 mM L-dihydroxyphenylalanine (L-DOPA) as substrate (Sigma Aldrich, St. Louis, USA) (Table 1) (Waldrop and Firestone 2004). Fluorimetric substrates (Glycosynth Ltd, Warrington, UK) were dissolved in 0.4 % methyl cellosolve (2-methoxyethanol; 0.1 % final concentration in the assay). For each soil sample, five replicate micro-plates were prepared and incubated at 2, 10, 22, 30 and 40 °C respectively.
For the fluorimetric assays, 2 g fresh soil (field moist weight basis) was added to 200 ml of 1 mM sodium azide (NaN3) solution and dispersed by stirring vigorously on a magnetic stir plate. After 5 min, and while stirring, 50 µl aliquots of soil suspension were removed using an 8-channel pipette and dispensed into a 96-well microplate containing 50 µl modified universal buffer solution (Tabatabai 1994) adjusted to soil pH. Each microplate included assay wells (soil solution, buffer and 100 µl of 200 µM MU substrate; 100 µM MU substrate in final solution), blank wells (soil solution, buffer and 100 µl of 1 mM NaN3) and quench wells (soil solution, buffer and 100 µl MU standard). There were eight analytical replicate wells for each assay, and control plates for each set of assays with the MU standards and no soil solution (to determine fluorescence from substrates and quenching by soil solution in assay plates). Microplates were incubated at each specified temperature for either 1 h (β-glucosidase, N-acetyl β-glucosaminidase, phosphomonoesterase) or 4 h (cellobiohydrolase, β-xylanase). Incubation times were based on preliminary assays to assess the linearity of the reaction over time. Following incubation, 50 µl of 0.5 M NaOH was added to each well and plates were immediately analyzed on a Fluostar Optima spectrofluorimeter (BMG Labtech, Offenburg, Germany) with excitation at 360 nm and emission at 450 nm.
For phenol oxidase assays, 1 g soil (oven-dry basis) was added to 100 ml of 5 mM bicarbonate buffer and stirred vigorously; 100 μl of 5 mM L-DOPA solution and 100 μl of soil solution were dispensed into a 96-well microplate. Control plates were made using 100 μl of 5 mM bicarbonate buffer and 100 μl aliquots of soil solution. There were 16 analytical replicates and controls per soil sample. Plates were analyzed on a Fluostar Optima spectrofluorometer (BMG Labtech, Offenburg, Germany), with phenol oxidase activity calculated as the increase in absorbance at 450 nm over 1 h.
Calculations and statistics
Enzyme activities
Enzyme activities were expressed on the basis of soil organic C to allow comparisons among sites with different organic C concentrations. Normalising enzyme activities to soil organic C is a standard approach in order to make comparisons among ecosystems on contrasting soils (Sinsabaugh et al. 2008), rather than to microbial C which can be highly variable among soils rich in clay or organic matter (Alessi et al. 2011). Hydrolytic enzyme activities, determined using MU substrates, were expressed in nmol MU min−1 g C−1. Phenol oxidase, determined using L-DOPA as a substrate, was expressed in mg diqc h−1 g C−1 (where diqc is the L-DOPA product 3-dihydroindole-5,6-quinone-2-carboxylate).
Determination of Q10 values
We determined the temperature sensitivity of maximum potential enzyme activity (V max ) by calculating Q 10 values as follows:
and
where k is the exponential rate at which V max increases with temperature (t) (Wallenstein et al. 2009). To calculate k (and thus Q 10) we used linear regression and included enzyme data determined between 2 °C and 40 °C. We only determined Q 10 values of enzyme activity during the exponential increase in activity with temperature according to Arrhenius kinetics (Wallenstein et al. 2009), prior to reaching any thermal optima of activity at which dynamics depart from Arrhenius kinetics (Schipper et al. 2014). The thermal optima of enzymes are widely associated with enzyme denaturation that begins to occur at temperatures above 40 °C (Price and Stevens 1999). To ensure that we accurately determined Q 10 values according to Arrhenius kinetics, for determination of Q 10 values for enzymes when V max at 40 °C was lower than activity at 30 °C (possibly due to enzyme denaturation at high temperature), we used data determined between 2 and 30 °C only.
The effects of assay temperature and elevation on enzyme activities were analysed using two-way ANOVA with ‘temperature’ and ‘elevation’ as factors, and ‘activity’ as the response variable. The effect of elevation alone on enzyme activities at each assay temperature was analysed using one-way ANOVA with ‘elevation’ as the factor and ‘activity’ as the response variable. The effects of elevation and other site/soil properties on the temperature sensitivity of enzyme activities (Q 10 of V max) were examined using Spearman’s correlations. The effect of enzyme class on the Q 10 of V max was analysed using one-way ANOVA with ‘enzymes class’ as the factor and ‘Q 10 of V max’ as the response variable. For one-way ANOVA analyses, pair-wise comparisons were performed using Tukey post hoc analyses. Data were log-transformed when model residuals were non-normally distributed. Significant interactions were determined at p ≤ 0.05. All statistical analyses were performed using R version 2.15 (R Core Team 2012).
Results
Total soil C and N concentrations increased with elevation across all sites (Table 2). Total soil C ranged from 10.3 % (at 1500 m asl) to 46.5 % (at 3030 m asl) and total soil N ranged from 0.91 % (at 1500 m asl) to 2.49 % (at 3400 m asl). Total soil P concentrations and soil pH did not vary with elevation. Soil P ranged from 0.71 mg P g−1 (at 2020 m asl) to 1.44 (at 1750 m asl) and soil pH ranged from 3.8 to 4.1 among sites.
All enzyme activities (V max) varied significantly with elevation and assay temperature (p < 0.001 for all comparisons; Fig. 1). The overall trend for all enzymes was a decrease in activity with elevation, which was approximately 100-fold after accounting for differences in soil C content among sites (Fig. 1; note log scale for enzyme activity). Enzyme activities generally increased with warmer incubation temperature for all enzyme classes (Fig. 1), although there were some exceptions. For example, there was an overall trend of greater enzyme activities at 10 °C than at 2 °C, but β-glucosidase activity was greater at 2 °C than at 10 °C for sites above 2500 m asl (Fig. 1). There was an overall trend of greater enzyme activities at 40 °C than at 30 °C, but there were some exceptions of greater phosphomonoesterase activities at 30 °C than at 40 °C (Fig. 1).
Maximum potential activities (V max) of enzymes, which act on carbon (β-glucosaminidase, cellobiohydrolase, β-xylanase, phenol oxidase), phosphorus (phosphomonoesterase) and nitrogen (N-acetyl β-glucosaminidase) in tropical montane forest soils. Data are for 10 sites at elevations ranging from 1500 to 3400 m asl, determined at temperatures ranging from 2 to 40 °C and at a standard substrate concentration (5 mM L-DOPA for phenol oxidase, or 100 µM MU for all other enzymes). All enzyme activities were determined at 5 temperatures (2, 10, 22, 30, 40 °C) and are shown on a log10 scale. Values are means with 1 standard error (n = 5 replicates, which represents the spatial variation within a 1 ha plot)
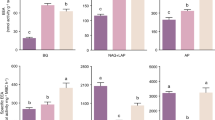
Linear models to determine the temperature sensitivities of enzyme activities (Q 10 of V max) (Eq. 3) were, on average, highly significant (R2 = 0.85 averaged across all enzymes and all sites; see Table S1 for R2 values for each enzyme at each site). The Q 10 of V max varied significantly with elevation except for N-acetyl β-glucosaminidase (p = 0.11) and phenol oxidase (p = 0.37). The Q 10 of V max for enzymes β-glucosidase and β-xylanase increased linearly with elevation (Table 3; Fig. 2). Although the Q 10 of V max for other enzymes were not significantly related to elevation, there were positive trends for cellobiohydrolase, phenol oxidase and phosphomonoesterase, but a negative trend for N-acetyl β-glucosaminidase (Table 3; Fig. 2). In relation to other site or soil properties, total N and total P were related to the Q 10 of V max for β-glucosidase only (ρ = 0.61 and 0.29), although these correlations were weak relative to elevation or MAT (ρ = 0.71 and 0.72).
The average Q 10 value of V max across all enzymes and sites was 1.7 ± 0.1, although there were differences in Q 10 values among sites and enzyme classes (Figs. 2, 3). Enzyme temperature sensitivities, when averaged across all sites, varied significantly among C-degrading enzymes, but not between N- or P-degrading enzymes (Fig. 3). The average Q 10 values of V max for C-degrading enzymes increased in the order: phenol oxidase <β-glucosidase <β-xylanase <cellobiohydrolase (Fig. 3). The average Q 10 value of V max for cellobiohydrolase was significantly higher than all other enzymes, while the Q 10 value of V max for β-glucosidase and phenol oxidase were significantly lower than all other enzymes (p < 0.05; all comparisons).
Discussion
Temperature-adaptation of iso-enzymes
The temperature sensitivities of activities for the enzymes β-glucosidase and β-xylanase were greater in higher elevation and colder sites (Fig. 2), partially supporting our first hypothesis of greater temperature sensitivity of enzymes from higher elevations. The temperature sensitivities of other enzymes were not significantly related to elevation, although there was a positive trend for five of the six enzymes under study. The apparent temperature acclimation of β-glucosidase and β-xylanase may be related to the importance of these enzymes as a rate-limiting step in cellulose and hemicellulose degradation, respectively. β-glucosidase cleaves β-1,4-glycosidic bonds between glucose molecules to yield free glucose for microbial assimilation, while β-xylanase degrades β-1,4-xylan, a polysaccharide abundant in plant cell walls. In the context of these functions, three mechanisms might explain the apparent temperature acclimation of β-glucosidase and β-xylanase: the presence of temperature-acclimated ‘iso-enzymes’, temperature-related changes in substrate supply, and/or rates of enzyme turnover.
Apparent thermal acclimation of enzyme activity may result from the presence of temperature-adapted iso-enzymes, which occur as an acclimation response of the enzyme itself or of the enzyme-producing microbial community. These iso-enzymes catalyse the same reaction but differ in chemical structure, enabling them to act more effectively under the ambient temperature. Temperature adapted iso-enzymes have been suggested to explain variation of enzymatic activity with seasonal temperature changes (Fenner et al. 2005; Koch et al. 2007; Trasar-Cepeda et al. 2007), across geographic temperature gradients (German et al. 2012; Olander and Vitousek 2000), and following experimental warming (Brzostek and Finzi 2012). Along a latitudinal gradient, the temperature sensitivity of β-glucosidase half-saturation constants (K m) was greater for soil from higher latitude (lower MAT) (German et al. 2012), while the activity of three enzymes (including β-glucosidase) in an alpine ecosystem had greater Q 10 values at colder seasonal temperatures (Koch et al. 2007). The strong evidence for acclimation of β-glucosidase in our study and others might be related to the importance of β-glucosidase as a rate-limiting step in cellulose decomposition (see above). Resource competition among decomposers is greater for labile C than recalcitrant C (Fontaine and Barot 2005), so we hypothesize that the associated increased selective pressure results in the synthesis of acclimated labile-C degrading iso-enzymes, including β-glucosidase.
Alternatively, the elevation-related differences in the temperature sensitivity of β-glucosidase and β-xylanase might be explained by increased substrate availability and rates of enzyme turnover at higher incubation temperatures. Changes in the turnover of different enzymes were reported in experimentally-warmed peatland soils, where increased release of phenolic compounds through the action of phenol oxidase had an inhibitory effect on the activity of hydrolytic enzymes, leading to an accumulation of dissolved organic C (Freeman et al. 2004). By the same mechanism, increased temperature might amplify Q 10 values in the C-rich montane tropical forest soils (Table 2). Phenolic compounds are less abundant in higher elevation soils along this tropical elevation gradient (Zimmermann et al. 2012), so warming of these soils may have an amplifying effect on the activity and turnover of hydrolytic enzymes, with the increased turnover of labile C.
The relative importance of these three mechanisms in regulating enzyme Q 10 responses is difficult to determine, especially given that MAT and substrate availability co-vary along the elevation gradient. However, we propose that the presence of temperature acclimated iso-enzymes explains the Q 10 responses, because changes in substrate availability or enzyme turnover rates cannot simultaneously explain the broad responses of other enzyme classes. For example, the Q 10 of all enzymes apart from β-glucosidase was not related to soil total C, N and P, while an inhibitory effect of phenol oxidase on the turnover of hydrolytic enzymes (Freeman et al. 2004) was not supported for any hydrolytic enzyme.
Temperature sensitivity across enzyme classes
In addition to our evidence for apparent acclimation of specific enzymes at different sites, we found consistent patterns in the thermal sensitivity of enzyme classes. For the hydrolytic enzymes under study, our results support our second hypothesis and follow kinetic theory because Q 10 values increased in the order β-glucosidase (Q 10 = 1.6 ± 0.05 standard error) < β-xylanase (Q 10 = 1.7 ± 0.03) < cellobiohydrolase (Q 10 = 2.0 ± 0.07) (Fig. 3). This pattern has been observed in other ecosystems; for example in temperate grassland soils, Trasar-Cepeda et al. (2007) found higher Q 10 values for cellulase compared to β-glucosidase. These findings support the considerable experimental evidence showing higher temperature sensitivity of complex C compound degradation (Craine et al. 2010; Frey et al. 2013; Knorr et al. 2005), including two studies performed along this elevation transect (Zimmermann et al.2010a, b).
However, kinetic theory is not consistently supported for phenol oxidase in this study, or for oxidative enzymes studied elsewhere (Steinweg et al. 2013; Trasar-Cepeda et al. 2007), suggesting different constraints on the temperature sensitivity of oxidative and hydrolytic enzymes. Phenol oxidase, which depolymerizes high molecular weight compounds such as lignin, had the lowest Q 10 value of 1.4 (±0.07). This value is identical to that reported in temperate peatland soils (Freeman et al. 2001) and within the range of Q10 values reported for oxidoreductases in temperate grassland soils (1.2–1.5) (Trasar-Cepeda et al. 2007). However, this general pattern contrasts with a different study of soils from arctic, temperate and tropical ecosystems, where the temperature sensitivity of enzyme activation energy was relatively low for peroxidase, but high for phenol oxidase (Steinweg et al. 2013). The low temperature sensitivity of oxidative enzymes reported in the majority of these studies, despite experimental evidence that soil organic matter degradation follows kinetic theory (Craine et al. 2010; Frey et al. 2013; Knorr et al. 2005), suggests that degradation of complex C compounds is limited by other factors in addition to the temperature response of oxidative enzymes. For example, the temperature sensitivity of oxidative degradation of complex C compounds may be regulated foremost by physical protection of substrates determined by macromolecular structure, soil mineralogy and soil pH, rather than kinetic attributes of enzymes (Sinsabaugh 2010; Zimmermann et al. 2012). However, inconsistency among these studies (Steinweg et al. 2013) also suggests that the determination of oxidative enzyme activity may be confounded by differences in soil properties (e.g. soil pH) or by the assay methodology itself (Sinsabaugh 2010). For example, laboratory assays using simple model substrates might not fully characterise the temperature sensitivity of naturally occurring complex C substrates, given the greater number of depolymerisation reactions required to degrade these substrates.
Generalisations of enzyme temperature sensitivities and climatic warming
There is increasing interest in the role of enzymes in soil organic matter cycling in a changing climate (Bradford et al. 2010; Henry 2012). Our findings suggest that enzyme-mediated reactions that determine rates of specific substrate cycling will broadly respond at an equivalent rate to temperature change along this elevation transect and across other similarly ranging temperature gradients. The range of Q 10 values of enzyme activities along our tropical elevation gradient were relatively constrained (1.4–2.0) and consistent with studies in other ecosystems: 1.5–3.0 in Arctic soils (Wallenstein et al. 2009), 1.5–2.5 in temperate forest soils (Baldrian et al. 2012), 1.5–2.7 in alpine soils (Koch et al. 2007) and an average of 1.5 in a temperate grassland (Trasar-Cepeda et al. 2007). This consistency suggests that some broad generalisations of enzyme temperature responses could be incorporated into soil C cycle models. For example, the average Q 10 of V max for both C-degrading and N and P-degrading enzymes was 1.7.
However, some specific enzymes exhibit local acclimation (Fig. 2) and while hydrolytic enzymes follow kinetic theory oxidative enzymes might not (Fig. 3), which limits the use of a single Q 10 value in modelling studies. Emerging evidence from our study and others (German et al. 2012) suggests that specific enzymes exhibit different temperature sensitivities in different environments, which may occur through interactions between temperature and soil properties or apparent temperature acclimation of the enzyme itself. Furthermore, although we found some evidence to support kinetic theory for hydrolytic enzymes, the low temperature sensitivity of phenol oxidase suggests that enzymatic processes are not the rate-limiting step in determining the thermal stability of more complex C molecules. We conclude that although we observe general behaviour in some enzyme classes, over-generalisation of enzyme temperature sensitivities could risk missing important overall responses in soil to change in temperature.
To more fully understand the consequences of temperature acclimated chemical reactions under field conditions, further studies are required using natural polymeric substrates. In addition, in situ studies should consider the long-term effects of temperature on substrate and/or nutrient availability, soil moisture and other biotic feedbacks that might influence enzyme activity and turnover. For example, field experiments have demonstrated warming effects on enzyme activities as a consequence of indirect effects on substrate supply (Baldrian et al. 2012; Brzostek et al. 2012; Brzostek and Finzi 2012; McDaniel et al. 2012), while differences in substrate supply may contribute to the local acclimation of enzymes along the elevation gradient studied here (Fig. 2). The response of enzymatic SOM degradation to temperature also depends on half-saturation (Km) constants, which may respond to temperature in a manner that offsets SOM losses associated with V max responses (German et al. 2012). Enzyme-specific thermal sensitivities have important implications for our understanding of the cycling of different nutrients and organic matter of different complexity under elevated temperatures (Allison et al. 2010; Billings and Ballantyne 2012). While more controlled experiments are required to resolve the mechanism involved, the apparent site-acclimation of specific enzyme Q 10 responses has important ecological consequences. For example, our findings for β-glucosidase and β-xylanase indicate high thermal vulnerability of soil C in tropical montane forests. Our findings suggest a long-term adaptive response of enzymes (or of the enzyme-producing microbial community) to temperature, although it remains to be seen whether, and the extent to which, this adaptive response will respond to short-term warming scenarios.
Conclusions
Along an Andean elevation gradient where MAT varies by 10 °C, we found constrained temperature sensitivities of soil enzyme activities (ranging from 1.4 to 2.0). Among the enzymes tested, the temperature sensitivity of β-glucosidase and β-xylanase activity increased with elevation, which suggests acclimation of these key enzymes involved in cellulose and hemicellulose degradation. The temperature sensitivities of different enzyme classes were also relatively constrained (both C, N and P-degrading enzymes had an average Q10 of 1.7). We detected higher temperature sensitivities for enzymes that hydrolyze more complex C compounds than for those hydrolyzing simpler C compounds. Our findings demonstrate that by assuming uniform temperature responses of different enzymes in Earth system models (which may or may not occur at broad scales), important localised enzyme-specific responses with potentially large regional consequences will be neglected. The acclimation of some important C-degrading enzymes along this Andean elevation gradient, for example, indicates that the large stocks of relatively chemically labile and physically unprotected soil C held in tropical montane ecosystems might be especially vulnerable to increased temperature.
References
Alessi DS, Walsh DM, Fein JB (2011) Uncertainties in determining microbial biomass C using the chloroform fumigation–extraction method. Chem Geol 280:58–64
Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3:336–340
Asner GP, Anderson CB, Martin RE, Knapp DE, Tupayachi R, Sinca F, Malhi Y (2014) Landscape-scale changes in forest structure and functional traits along an Andes-to-Amazon elevation gradient. Biogeosciences 11:843–856
Baldrian P, Šnajdr J, Merhautová V, Dobiášová P, Cajthaml T, Valášková V (2012) Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biol Biochem 56:60–68
Billings SA, Ballantyne F (2012) How interactions between microbial resource demands, soil organic matter stoichiometry, and substrate reactivity determine the direction and magnitude of soil respiratory responses to warming. Glob Change Biol 19:90–102
Bradford MA (2013) Thermal adaptation of decomposer communities in warming soils. Front Microbiol 4:333. doi:10.3389/fmicb.2013.00333
Bradford MA, Watts BW, Davies CA (2010) Thermal adaptation of heterotrophic soil respiration in laboratory microcosms. Glob Change Biol 16:1576–1588
Brzostek ER, Finzi AC (2012) Seasonal variation in the temperature sensitivity of proteolytic enzyme activity in temperate forest soils. J Geophys Res. doi:10.1029/2011JG001688
Brzostek ER, Blair JM, Dukes JS, Frey SD, Hobbie SE, Melillo JM, Mitchell RJ, Pendall E, Reich PB, Shaver GR, Stefanski A, Tjoelker MG, Finzi AC (2012) The effect of experimental warming and precipitation change on proteolytic enzyme activity: positive feedbacks to nitrogen availability are not universal. Glob Change Biol 18:2617–2625
Burns R, Staunton S (2013) Special issue: interactions of soil minerals with organic components and microorganisms VII and enzymes in the environment IV. Soil Biol Biochem 56:1–2
Carlotto V, Gil W, Cardenas J, Chavez R (1996) Mapa Geologico del Cuadrangula de Calca (27-s). In: Ministerio de engergia y minas Instituto geologico minero y metalurgico (INGEMMET)
Clark KE, Hilton RG, West AJ, Malhi Y, Gröcke DR, Bryant CL, Ascough PL, Robles Caceres A, New M (2013) New views on “old” carbon in the Amazon River: insight from the source of organic carbon eroded from the Peruvian Andes. Geochem Geophys Geosyst 14:1644–1659
Conant RT, Ryan MG, Ågren GI, Birge HE, Davidson EA, Eliasson PE, Evans SE, Frey SD, Giardina CP, Hopkins FM, Hyvönen R, Kirschbaum MUF, Lavallee JM, Leifeld J, Parton WJ, Megan Steinweg J, Wallenstein MD, Martin Wetterstedt JÅ, Bradford MA (2011) Temperature and soil organic matter decomposition rates—synthesis of current knowledge and a way forward. Glob Change Biol 17:3392–3404
Craine JM, Fierer N, McLauchlan KK (2010) Widespread coupling between the rate and temperature sensitivity of organic matter decay. Nat Geosci 3:854–857
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Dong YW, Somero GN (2009) Temperature adaptation of cytosolic malate dehydrogenases of limpets (genus Lottia): differences in stability and function due to minor changes in sequence correlate with biogeographic and vertical distributions. J Exp Biol 212:169–177
Feeley KJ, Silman MR, Bush MB, Farfan W, Cabrera KG, Malhi Y, Meir P, Revilla NS, Quisiyupanqui MNR, Saatchi S (2011) Upslope migration of Andean trees. J Biogeogr 38:783–791
Fenner N, Freeman C, Reynolds B (2005) Observations of a seasonally shifting thermal optimum in peatland carbon-cycling processes; implications for the global carbon cycle and soil enzyme methodologies. Soil Biol Biochem 37:1814–1821
Fierer N, Colman BP, Schimel JP, Jackson RB (2006) Predicting the temperature dependence of microbial respiration in soil: a continental-scale analysis. Glob Biogeochem Cycles. doi:10.1029/2005GB002644
Fontaine S, Barot S (2005) Size and functional diversity of microbe populations control plant persistence and long-term soil carbon accumulation. Ecol Lett 8:1075–1087
Freeman C, Ostle N, Kang H (2001) An enzymic ‘latch’ on a global carbon store. Nature 409:149
Freeman C, Ostle NJ, Fenner N, Kang H (2004) A regulatory role for phenol oxidase during decomposition in peatlands. Soil Biol Biochem 36:1663–1667
Frey SD, Lee J, Melillo JM, Six J (2013) The temperature response of soil microbial efficiency and its feedback to climate. Nat Clim Change 3:395–398
German DP, Marcelo KRB, Stone MM, Allison SD (2012) The Michaelis-Menten kinetics of soil extracellular enzymes in response to temperature: a cross-latitudinal study. Glob Change Biol 18:1468–1479
Girardin CAJ, Malhi Y, Aragao LEOC, Mamani M, Huaraca Huasco W, Durand L, Feeley KJ, Rapp J, Silva-Espejo JE, Silman M, Salinas N, Whittaker RJ (2010) Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes. Glob Change Biol 16:3176–3192
Henry HAL (2012) Soil extracellular enzyme dynamics in a changing climate. Soil Biol Biochem 47:53–59
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, New York
Jobbagy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:423–436
Knorr W, Prentice IC, House JI, Holland EA (2005) Long-term sensitivity of soil carbon turnover to warming. Nature 433:298–301
Koch O, Tscherko D, Kandeler E (2007) Temperature sensitivity of microbial respiration, nitrogen mineralization, and potential soil enzyme activities in organic alpine soils. Glob Biogeochem Cycles. doi:10.1029/2007gb002983
McDaniel MD, Kaye JP, Kaye MW (2012) Increased temperature and precipitation had limited effects on soil extracellular enzyme activities in a post-harvest forest. Soil Biol Biochem 56:90–98
Moser G, Leuschner C, Hertel D, Graefe S, Soethe N, Iost S (2011) Elevation effects on the carbon budget of tropical mountain forests (S Ecuador): the role of the belowground compartment. Glob Change Biol 17:2211–2226
Nottingham AT, Turner BL, Chamberlain PM, Stott AW, Tanner EVJ (2011) Priming and microbial nutrient limitation in lowland tropical forest soils of contrasting fertility. Biogeochemistry 111:219–237
Nottingham AT, Turner BL, Whitaker J, Ostle N, McNamara NP, Bardgett RD, Salinas N, Meir P (2015) Soil microbial nutrient constraints along a tropical forest elevation gradient: a belowground test of a biogeochemical paradigm. Biogeosciences 12:6071–6083
Olander LP, Vitousek PM (2000) Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 49:175–190
Price N, Stevens L (1999) Fundamentals of enzymology: cell and molecular biology of catalytic proteins. Oxford University Press, New York
Quesada CA, Lloyd J, Schwarz M, Patiño S, Baker TR, Czimczik C, Fyllas NM, Martinelli L, Nardoto GB, Schmerler J, Santos AJB, Hodnett MG, Herrera R, Luizão FJ, Arneth A, Lloyd G, Dezzeo N, Hilke I, Kuhlmann I, Raessler M, Brand WA, Geilmann H, Moraes Filho JO, Carvalho FP, Araujo Filho RN, Chaves JE, Cruz Junior OF, Pimentel TP, Paiva R (2010) Variations in chemical and physical properties of Amazon forest soils in relation to their genesis. Biogeosciences 7:1515–1541
Rapp JM, Silman MR (2012) Diurnal, seasonal, and altitudinal trends in microclimate across a tropical montane cloud forest. Clim Res 55:17–32
R Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Schipper LA, Hobbs JK, Rutledge S, Arcus VL (2014) Thermodynamic theory explains the temperature optima of soil microbial processes and high Q(10) values at low temperatures. Glob Change Biol 20:3578–3586
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Somero GN (2004) Adaptation of enzymes to temperature: searching for basic “strategies”. Comp Biochem Physiol B: Biochem Mol Biol 139:321–333
Steinweg JM, Jagadamma S, Frerichs J, Mayes MA (2013) Activation energy of extracellular enzymes in soils from different biomes. PLoS One 8:e59943
Tabatabai MA (1994) Soil enzymes. In: Weaver R et al (eds) Methods of soil analysis. Part 2. Microbiological and biochemical properties. SSSA, Madison, pp 778–833
Trasar-Cepeda C, Gil-Sotres F, Leiros MC (2007) Thermodynamic parameters of enzymes in grassland soils from Galicia, NW Spain. Soil Biol Biochem 39:311–319
Turner BL, Romero TE (2010) Stability of hydrolytic enzyme activity and microbial phosphorus during storage of tropical rain forest soils. Soil Biol Biochem 42:459–465
van de Weg MJ, Meir P, Grace J, Atkin OK (2009) Altitudinal variation in leaf mass per unit area, leaf tissue density and foliar nitrogen and phosphorus content along an Amazon-Andes gradient in Peru. Plant Ecol Divers 2:243–254
van de Weg MJ, Meir P, Williams M, Girardin C, Malhi Y, Silva-Espejo J, Grace J (2014) Gross primary productivity of a high elevation tropical montane cloud forest. Ecosystems 17:751–764
Vuille M, Bradley RS (2000) Mean annual temperature trends and their vertical structure in the tropical Andes. Geophys Res Lett 27:3885–3888
Waldrop MP, Firestone MK (2004) Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak-woodland plant communities. Oecologia 138:275–284
Wallenstein MD, McMahon SK, Schimel JP (2009) Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Change Biol 15:1631–1639
Wallenstein M, Allison S, Ernakovich J, Steinweg JM, Sinsabaugh R (2011) Controls on the temperature sensitivity of soil enzymes: a key driver of in situ enzyme activity rates. In: Shukla G, Varma A (eds) Soil enzymology, soil biology. Springer, Berlin, pp 245–258
Whitaker J, Ostle N, Nottingham AT, Ccahuana A, Salinas N, Bardgett RD, Meir P, McNamara NP (2014) Microbial community composition explains soil respiration responses to changing carbon inputs along an Andes-to-Amazon elevation gradient. J Ecol 102:1058–1071
Wieder WR, Bonan GB, Allison SD (2013) Global soil carbon projections are improved by modelling microbial processes. Nat Clim Change 3:909–912
Zimmermann M, Meir P, Bird MI, Malhi Y, Ccahuana AJQ (2009) Climate dependence of heterotrophic soil respiration from a soil-translocation experiment along a 3000 m tropical forest altitudinal gradient. Eur J Soil Sci 60:895–906
Zimmermann M, Meir P, Bird MI, Malhi Y, Ccahuana AJQ (2010a) Temporal variation and climate dependence of soil respiration and its components along a 3000 m altitudinal tropical forest gradient. Glob Biogeochem Cycles 24(4):GB4012
Zimmermann M, Meir P, Bird MI, Malhi Y, Ccahuana AJQ (2010b) Temporal variation and climate dependence of soil respiration and its components along a 3000 m altitudinal tropical forest gradient. Glob Biogeochem Cycles 24
Zimmermann M, Meir P, Silman MR, Fedders A, Gibbon A, Malhi Y, Urrego DH, Bush MB, Feeley KJ, Garcia KC, Dargie GC, Farfan WR, Goetz BP, Johnson WT, Kline KM, Modi AT, Rurau NMQ, Staudt BT, Zamora F (2010c) No differences in soil carbon stocks across the tree line in the Peruvian Andes. Ecosystems 13:62–74
Zimmermann M, Leifeld J, Conen F, Bird MI, Meir P (2012) Can composition and physical protection of soil organic matter explain soil respiration temperature sensitivity? Biogeochemistry 107:423–436
Acknowledgments
This study is a product of the Andes Biodiversity and Ecosystem Research Group consortium (www.andesconservation.org) and was financed by the UK Natural Environment Research Council (NERC) (grant numbers NE/G018278/1, NE/F002149/1), and also supported by ARC grant FT110100457 to PM and a European Union Marie-Curie Fellowship FP7-2012-329360 to ATN. We thank the Asociacion para la Conservacion de la Cuenca Amazonica (ACCA) in Cusco and the Instituto Nacional de Recursos Naturales (INRENA) in Lima for access to the study sites. For their logistical support we thank Dr. Eric Cosio and Eliana Esparza Ballón at Pontificia Universidad Católica del Perú (PUCP). For their support in the laboratory we thank Tania Romero and Dayana Agudo. For their support in the field we thank Adan J.Q. Ccahuana, Walter H. Huasco, Javier E. S. Espejo and many others too numerous to mention here.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Kate Lajtha.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nottingham, A.T., Turner, B.L., Whitaker, J. et al. Temperature sensitivity of soil enzymes along an elevation gradient in the Peruvian Andes. Biogeochemistry 127, 217–230 (2016). https://doi.org/10.1007/s10533-015-0176-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-015-0176-2