Abstract
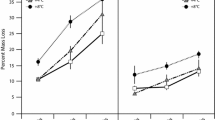
Studies conducted across northern Europe and North America have shown increases in dissolved organic carbon (DOC) in aquatic systems in recent decades. While there is little consensus as to the exact mechanisms for the increases in DOC, hypotheses converge on such climate change factors as warming, increased precipitation variability, and changes in atmospheric deposition. In this study, we tested the effects of warming on peat porewater composition by actively warming a peatland with infrared lamps mounted 1.24 m above the peat surface for 3 years. Mean growing season peat temperatures in the warmed plots (n = 5) were 1.9 ± 0.4 °C warmer than the control plots at 5 cm depth (t statistic = 5.03, p = 0.007). Mean porewater DOC concentrations measured throughout the growing season were 15 % higher in the warmed plots (73.4 ± 3.2 mg L−1) than in the control plots (63.7 ± 2.1 mg L−1) at 25 cm (t = 4.69, p < 0.001). Furthermore, DOC from the warmed plots decayed nearly twice as fast as control plot DOC in laboratory incubations, and exhibited lower aromaticity than control plot porewater (reduction in SUVA254 in heated plots compared with control plots). Dissolved organic nitrogen (DON) concentrations tracked DOC patterns as expected, but the amount of dissolved N per unit C decreased with warming. Previous work has shown that warming increased net primary production at this site, and together with measured increases in the activities of chitinases and glucosidases we suggest that the increased DOC concentrations observed with warming were derived in part from microbial-plant interactions in the rhizosphere. We also detected more nitrogen containing compounds with higher double bond equivalents (DBE) unique to the warmed plots, within the pool of biomolecules able to deprotonate (16 % of all compounds identified using ultrahigh resolution ion electrospray mass spectrometry); we suggest these compounds could be the products of increased plant, microbial, and enzyme activity occurring with warming. With continued warming in peatlands, an increase in relatively labile DOC concentrations could contribute to dissolved exports of DOC in runoff, and would likely contribute to the pool of efficient electron donors (and acceptors) in the production of CO2 and CH4 in terrestrial and aquatic environments.








Similar content being viewed by others
References
Aitkenhead JA, McDowell WH (2000) Soil C: N ratio as a predictor of annual riverine DOC flux at local and global scales. Glob Biogeochem Cycles 14(1):127–138
Albert DA, Wilcox DA, Ingram JW, Thompson TA (2005) Hydrogeomorphic classification for Great Lakes coastal wetlands. J Great Lakes Res 31:129–146
Alewell C, Paul S, Storck FR (2008) Co-regulation of redox processes in freshwater wetlands as a function of organic matter availability? Sci Total Environ 404:335–342
Allison SD, Treseder KK (2008) Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob Chang Biol 14:2898–2909
Alvarez-Cobelas M, Angeler DG, Sanchez-Carrillo S, Almendros G (2012) A worldwide view of organic carbon export from catchments. Biogeochemistry 107(1–3):275–293
Battin TJ, Luyssaert S, Kaplan LA, Aufdenkampe AK, Richter A, Tranvik LJ (2009) The boundless carbon cycle. Nat Geosci 2:598–600
Benner R (2003) Molecular indicators of the bioavailability of dissolved organic matter. In: Findlay SEG, Sinsabaugh RL (eds) Aquatic ecosystems: interactivity of dissolved organic matter. Academic Press, San Diego, pp 121–135
Blakney GT, Robinson DE, Ly NV, Kelleher NL, Hendrickson CL, Marshall AG (2005) Predator: PCI data station for FT-ICR mass spectrometry. 53rd American Society of Mass Spectrometry Annual Conference on Mass Spectrometry & Allied Topics, San Antonio
Blodau C, Basiliko N, Moore TR (2004) Carbon turnover in peatland mesocosms exposed to different water table levels. Biogeochemistry 67(3):331–351
Boisvert E (2009) Initiation and development of three lake superior costal peatlands. MS Thesis, Michigan Technological University, Houghton, p 77
Borken W, Ahrens B, Schulz C, Zimmermann L (2011) Site-to-site variability and temporal trends of DOC concentrations and fluxes in temperate forest soils. Glob Chang Biol 17(7):2428–2443
Bourbonniere RA (1989) Distribution patterns of dissolved organic matter fractions in natural waters from eastern Canada. Org Geochem 14:97–107
Bukaveckas PA, Robbins-Forbes M (2000) Role of dissolved organic matter in the attenuation of photosynthetically active and ultraviolet radiation in Adirondack lakes. Freshw Biol 43:339–354
Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013) Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem 58:216–234
Butler SM, Melillo JM, Johnson JE, Mohan J, Steudler PA, Lux H, Burrows E, Smith RM, Vario CL, Scott L, Hill TD, Aponte N, Bowles F (2012) Soil warming alters nitrogen cycling in a New England forest: implications for ecosystem function and structure. Oecologia 168:819–828
Caldwell B (2005) Enzyme activities as a component of soil biodiversity: a review. Pedobiologia 49:637–644
Chanton JP, Glaser PH, Chasar LS, Brudige DJ, Hines ME, Siegel DI, Tremblay LB, Cooper WT (2008) Radiocarbon evidence for the importance of surface vegetation on fermentation and methanogenesis in contrasting types of boreal peatlands. Glob Biogeochem Cycles 22:GB4022. doi:10.1029/2008GB003274
Christ MJ, David MB (1996) Temperature and moisture effects on the production of dissolved organic carbon in a Spodosol. Soil Biol Biochem 28:1191–1199
Clark JM, Ashley D, Wagner M, Chapman PJ, Lane SN, Evans CD, Heathwaite AL (2009) Increased temperature sensitivity of net DOC production from ombrotrophic peat due to water table draw-down. Glob Chang Biol 15:794–807
Clark JM, Bottrell SH, Evans CD, Monteith DT, Bartlett R, Rose R, Newton RJ, Chapman PJ (2010) The importance of the relationship between scale and process in understanding long-term DOC dynamics. Sci Total Environ. doi:10.1016/j.scitotenv.2010.02.046
Clemmensen KE, Michelsen A, Jonasson S, Shaver GR (2006) Increased ectomycorrhizal fungal abundance after long-term fertilization and warming of two arctic tundra ecosystems. New Phytol 171:391–404
Cody RP, Smith JK (1997) Applied statistics and the SAS programming language. Prentice-Hall, Englewood Cliffs
Dalva M, Moore TR (1991) Sources and sinks of dissolved organic carbon in a forested swamp catchment. Biogeochemistry 15:1–19
Driscoll CT, Driscoll KM, Roy KM, Mitchell MJ (2003) Chemical response of lakes in the Adirondack region of New York to declines in acidic deposition. Environ Sci Technol 37:2036–2042
Evans CD, Monteith DT, Cooper DM (2005) Long-term increases in surface water dissolved organic carbon: observations, possible causes and environmental impacts. Environ Pollut 137:55–71
Evans CD, Jones TG, Burden A, Ostle N, Zielinski P, Cooper MDA, Peacock M, Clark JM, Oulehle F, Cooper D, Freeman C (2012) Acidity controls on dissolved organic carbon mobility in organic soils. Glob Chang Biol 18:3317–3331
Fenner N, Freeman C, Lock MA, Harmens H, Reynolds B, Sparks T (2007) Interactions between elevated CO2 and warming could amplify DOC exports from peatland catchments. Environ Sci Technol 41:3146–3152
Freeman C, Evans CD, Monteith DT (2001a) Export of organic carbon from peat soils. Nature 412:785
Freeman C, Ostle N, Kang H (2001b) An enzymic ‘latch’ on a global carbon store. Nature 409:149–150
Freeman C, Fenner N, Ostle NJ, Kang H, Dowrick DJ, Reynolds B, Lock MA, Sleep D, Hughes S, Hudson J (2004) Export of dissolved organic carbon from peatlands under elevated carbon dioxide levels. Nature 430:195–198
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43(7):1387–1397
Gonet SS, Debska B (1998) Properties of humic acids developed during humification process of post-harvest plant residues. Environ Int 24:603–608
Guggenberger G, Zech W, Schulten H (1994) Formation and mobilization of dissolved organic matter: evidence form chemical structural studies of organic matter fractions in acid forest floor solutions. Org Geochem 21:51–66
Hatcher PG, Spiker EC (1988) Selective degradation of plant biomolecules. In: Frimmel FH, Christman RF (eds) Humic substances and their role in the environment. Wiley, New York, pp 59–74
Hedges JI (1990) Compositional indicators of organic acid sources and reactions in natural environments. In: Perdue EM, Gjessing ET (eds) Organic acids in aquatic ecosystems. Wiley, New York, pp 43–64
Heikkinen K (1994) Organic matter, iron and nutrient transport and nature of dissolved organic matter in the drainage basin of a boreal humic river in northern Finland. Sci Total Environ 152:81–89
Heitmann T, Goldhammer T, Beer J, Blodau C (2007) Electron transfer of dissolved organic matter and its potential significance for anaerobic respiration in a northern bog. Glob Chang Biol 13:1771–1785
Hessen DO (1992) Dissolved organic carbon in a small humic lake: effects on bacterial production and respiration. Hydrobiologia 229:115–123
Höll BS, Fiedler S, Jungkunst HF, Kalbitz K, Freibauer A, Drosler M, Stahr K (2009) Characteristics of dissolved organic matter following 20 years of peatland restoration. Sci Total Environ 408:78–83
Hoppe HG (1991) Microbial extracellular enzyme activity: a new key parameter in aquatic ecology. In: Chrost RJ (ed) Microbial enzymes in aquatic environments. Springer, Berlin, pp 60–83
Hughey CA, Hendrickson CL, Rodgers RP, Marshall AG, Qian KN (2001) Kendrick mass defect spectrum: a compact visual analysis for ultrahigh-resolution broadband mass spectra. Anal Chem 73(19):4676–4681
Jassey VEJ, Chiapusio G, Gilbert D, Toussaint ML, Binet P (2012) Phenoloxidase and peroxidase activities in Sphagnum-dominated peatland in a warming climate. Soil Biol Biochem 46:49–52
Jassey VEJ, Chiapusio G, Binet P, Buttler A, Laggoun-Defarge F, Delarue F, Bernard N, Mitchell EAD, Toussaint ML, Francez AJ, Gilbert D (2013) Above- and belowground linkages in Sphagnum peatland: climate warming affects plant–microbial interactions. Glob Chang Biol 19(3):811–823
Joanisse GD, Bradley RL, Preston CM, Munson AD (2007) Soil enzyme inhibition by condensed litter tannins may drive ecosystem structure and processes: the case of Kalmia angustifolia. New Phytol 175:535–546
Johansson EM, Fransson PMA, Finlay RD, van Hees PAW (2009) Quantitative analysis of soluble exudates produced by ectomycorrhizal roots as a response to ambient and elevated CO2. Soil Biol Biochem 41(6):1111–1116
Johnson CP, Pypker TG, Hribljan JA, Chimner RA (2013) Open top chambers and infrared lamps: a comparison of heating efficacy and CO2/CH4 dynamics in a Northern Michigan Peatland. Ecosystems 16(5):736–748
Judd KE, Kling GW (2002) Production and export of dissolved C in arctic tundra mesocosms: the roles of vegetation and water flow. Biogeochemistry 60(3):213–234
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165(4):277–304
Kalbitz K, Schmerwitz J, Schwesig D, Matzner E (2003) Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 113(3–4):273–291
Kane ES, Valentine DW, Michaelson GJ, Fox JD, Ping C-L (2006) Controls over pathways of carbon efflux from soils along climate and black spruce productivity gradients in interior Alaska. Soil Biol Biochem 38:1438–1450
Kane ES, Turetsky MR, Harden JW, McGuire AD, Waddington JM (2010) Seasonal ice and hydrologic controls on dissolved organic carbon and nitrogen concentrations in a boreal-rich fen. J Geophys Res 115:G04012. doi:10.1029/2010JG001366
Kang H, Freeman C, Park SS, Chun J (2005) N-Acetylglucosaminidase activities in wetlands: a global survey. Hydrobiologia 532:103–110
Keller JK, Weisenhorn PB, Megonigal JP (2009) Humic acids as electron acceptors in wetland decomposition. Soil Biol Biochem 41:1518–1522
Koch BP, Dittmar T (2006) From mass to structure: an aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun Mass Spectrom 20:926–932
Koch BP, Witt MR, Engbrodt R, Dittmar T, Kattner G (2005) Molecular formulae of marine and terrigenous dissolved organic matter detected by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Geochim Cosmochim Acta 69(13):3299–3308
Koch O, Tscherko D, Kandeler E (2007) Temperature sensitivity of microbial respiration, nitrogen mineralization, and potential soil enzyme activities in organic alpine soils. Glob Biogeochem Cycles 21(4):GB4017
Kolka RK, Grigal DF, Nater EA, Verry ES (2001) Hydrologic cycling of mercury and organic carbon in a forested upland-bog watershed. Soil Sci Soc Am J 65:897–905
Kujawinski EB (2002) Electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry (ESI FT-ICR MS): characterization of complex environmental mixtures. Environ Forensics 3(3–4):207–216
Kullberg A, Bishop KH, Hargeby A, Jansson M, Petersen RC (1993) The ecological significance of dissolved organic-carbon in acidified waters. Ambio 22:331–337
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165:382–396
Littell RC, Milliken GA, Stroup WA, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. SAS Institute Inc, Cary
Liu Z, Sleighter RL, Zhong J, Hatcher PG (2011) The chemical changes of DOM from black waters to coastal marine waters by HPLC combined with ultrahigh resolution mass spectrometry. Estuar Coast Shelf Sci 92:205–216
Mazzoleni LR, Ehrmann BM, Shen XH, Marshall AG, Collett JL (2010) Water-soluble atmospheric organic matter in fog: exact masses and chemical formula identification by ultrahigh-resolution Fourier transform ion cyclotron resonance mass spectrometry. Environ Sci Technol 44(10):3690–3697
McDowell WH, Likens G (1988) Origin, composition, and flux of dissolved organic carbon in the Hubbard Brook valley. Ecol Monogr 58:177–195
McKnight DM, Aiken GR (1998) Sources and age of aquatic humus. In: Hessen DO, Tranvik LJ (eds) Aquatic humic substances—ecology and biogeochemistry. Ecological studies 133. Springer, Berlin, pp 9–40
McManus JP, Davis KG, Beart JE, Gaffey SH, Lilley TH, Haslam E (1985) Polyphenol interactions. Part 1. Introduction. Some observations on the reversible complexation of polyphenols with proteins and polysaccharides. J Chem Soc Perkin Trans II 2(9):1429–1438
Melillo J, Butler S, Johnson J, Mohan J, Steudler P, Lux H, Burrows E, Bowles F, Smith R, Scott L, Vario C, Hill T, Burton A, Zhou Y, Tang J (2011) Soil warming, carbon–nitrogen interactions, and forest carbon budgets. Proc Natl Acad Sci USA 8(23):9508–9512
Michaelson GJ, Ping C-L, Kling GW, Hobbie JE (1998) The character and bioavailability of dissolved organic matter at thaw and in spring runoff waters of the arctic tundra north slope. J Geophys Res 103:28939–28946
Michalzik B, Kalbitz K, Park JH, Solinger S, Matzner E (2001) Fluxes and concentrations of dissolved organic carbon and nitrogen—a synthesis for temperate forests. Biogeochemistry 52:173–205
Monteith DT, Stoddard JL, Evans CD, de Wit HA, Forsius M, Hogasen T, Wilander A, Skjelkvale BL, Jeffries DS, Vuorenmaa J, Keller B, Kopacek J, Vesely J (2007) Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 450:537–540
Moore TR (2003). Dissolved organic carbon in a northern boreal landscape. Glob Biogeochem Cycles 17, 109. doi:10.1029/2003GB002050
Moore TR, Dalva M (2001) Some controls on the release of dissolved organic carbon by plant tissues and soils. Soil Sci 166(1):38–47
Moore TR, Pare D, Boutin R (2008) Production of dissolved organic carbon in Canadian forest soils. Ecosystems 11:740–751
Müller-Wegener U (1988) Interaction of humic substances with biota. In: Frimmel FH, Christman RF (eds) Humic substances and their role in the environment. Wiley, New York, pp 179–192
Münster U, de Haan H (1998) The role of microbial extracellular enzymes in the transformation of dissolved organic matter in humic waters. In: Hessen DO, Tranvik LJ (eds) Aquatic humic substances—ecology and biogeochemistry. Ecological studies 133. Springer, Berlin, pp 199–258
Nannipieri P, Kandeler E, Ruggiero P (2002) Enzyme activities and microbiological and biochemical processes in soil. In: Burns RG, Dick RP (eds) Enzymes in the environment. Marcel Dekker, New York, pp 1–34
Neff JC, Hooper DU (2002) Vegetation and climate controls on potential CO2, DOC and DON production in northern latitude soils. Glob Chang Biol 8:872–884
Park JH, Kalbitz K, Matzner E (2002) Resource control on the production of dissolved organic carbon and nitrogen in a deciduous forest floor. Soil Biol Biochem 34(6):813–822
Pastor J, Solin J, Bridgham SD, Updegraff K, Harth C, Weishampel P, Dewey B (2003) Global warming and the export of dissolved organic carbon from boreal peatlands. Oikos 100(2):380–386
Porcal P, Koprivnjak J-F, Molot LA, Dillon PJ (2009) Humic substances—part 7: the biogeochemistry of dissolved organic carbon and its interactions with climate change. Environ Sci Pollut Res 16:714–726
Preston MD, Eimers MC, Watmough SA (2011) Effect of moisture and temperature variation on DOC release from a peatland: conflicting results from laboratory, field and historical data analysis. Sci Total Environ. doi:10.1016/j.scitotenv.2010.12.027
Putman AL, Offenberg JH, Fisseha R, Kundu S, Rahn TA, Mazzoleni LR (2012) Ultrahigh-resolution FT-ICR mass spectrometry characterization of a-pinene ozonolysis SOA. Atmos Environ Part A 46(1):164–172
Rapalee G, Trumbore SE, Davidson EA, Harden JW, Veldhuis H (1998) Soil carbon stocks and their rates of accumulation and loss in a boreal forest landscape. Glob Biogeochem Cycles 12(4):687–701
Remucal CK, Cory RM, Sander M, McNeill K (2012) Low molecular weight components in an aquatic humic substance as characterized by membrane dialysis Orbitrap mass spectrometry. Environ Sci Technol 46:9350–9359
Roulet N, Moore TR (2006) Environmental chemistry—browning the waters. Nature 444(7117):283–284
Scott MJ, Jones MN, Woof C, Tipping E (1998) Concentrations and fluxes of dissolved organic carbon in drainage water from an upland peat system. Environ Int 24:537–546
Shackle VJ, Freeman C, Reynolds B (2000) Carbon supply and the regulation of enzyme activity in constructed wetlands. Soil Biol Biochem 32:1935–1940
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404
Sinsabaugh RL, Reynolds H, Long TM (2000) Rapid assay for amidohydrolase (urease) activity in environmental samples. Soil Biol Biochem 32:2095–2097
Sinsabaugh RL, Gallo ME, Lauber C, Waldrop MP, Zak DR (2005) Extracellular enzyme activities and soil organic matter dynamics for northern hardwood forests receiving simulated nitrogen deposition. Biogeochemistry 75(2):201–215
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Creshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Spencer CM, Cai Y, Martin R, Gaffney SH, Goulding PN, Magnolato D, Lilley TH, Haslam E (1988) Polyphenol complexation—some thoughts and observations. Phytochemistry 27:2397–2409
Stenson AC, Marshall AG, Cooper WT (2003) Exact masses and chemical formulas of individual Suwannee River fulvic acids from ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectra. Anal Chem 75(6):1275–1284
Strack M, Waddington JM, Bourbonniere RA, Buckton EL, Shaw K, Whittington P, Price JP (2008) Effect of water table drawdown on peatland dissolved organic carbon export and dynamics. Hydrol Process 22:3373–3385
Strack M, Toth K, Bourbonniere R, Waddington JM (2011) Dissolved organic carbon production and runoff quality following peatland extraction and restoration. Ecol Eng 37:1998–2008
Sucker C, Krause K (2010) Increasing dissolved organic carbon concentrations in freshwaters: what is the actual driver? iForest 3:106–108
Tegen I, Dorr H (1996) Mobilization of cesium in organic rich soils: correlation with production of dissolved organic carbon. Water Air Soil Pollut 88:133–144
Tfaily MM, Hamdan R, Corbett JE, Chanton JP, Glaser PH, Cooper WT (2013) Investigating dissolved organic matter decomposition in northern peatlands using complimentary analytical techniques. Geochimica et Cosmochimica Acta 112:116–129
Thurman EM (1985) Organic geochemistry of natural waters. Nijhoff/Junk, Boston
Tipping E, Woof C, Rigg E, Harrison AF, Inneson P, Taylor K, Benham D, Poskitt J, Rowland AP, Bol R, Harkness DD (1999) Climatic influences on the leaching of dissolved organic matter from upland UK moorland soils, investigated by a field manipulation experiment. Environ Int 25:83–95
Tranvik LJ, Jansson M (2002) Climate change—terrestrial export of organic carbon. Nature 415(6874):861–862
Urban NR, Gorham E, Underwood JK, Martin FB, Ogden JG (1990) Chemical processes controlling concentrations of Al, Fe, and Mn in Nova Scotia lakes. Limnol Oceanogr 35(7):1516–1534
Urban N, Verry ES, Eisenreich S, Grigal DF, Sebestyen SD (2011) Element cycling in upland/peatland watersheds. In: Kolka RK, Sebestyen SD, Verry ES, Brooks KN (eds) Peatland biogeochemistry and watershed hydrology at the marcell experimental forest. CRC Press, Boca Raton, pp 213–243
Visser SA (1983) Application of Van Krevelen’s graphical–statistical method for the study of aquatic humic material. Environ Sci Technol 17:412–417
Wallenstein MD, McMahon SK, Schimel JP (2009) Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Chang Biol 15:1631–1639
Wallenstein M, Allison SD, Ernakovich J, Steinweg JM, Sinsabaugh RL (2011) Controls on the temperature sensitivity of soil enzymes: a key driver of in situ enzyme activity rates. In: Shukla G, Varma A (eds) Soil enzymology. Springer, Berlin, pp 245–258
Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37(20):4702–4708
Wickland KP, Neff JC, Aiken GR (2007) Dissolved organic carbon in Alaskan boreal forest: sources, chemical characteristics, and biodegradability. Ecosystems 10:1323–1340
Worrall F, Burt T, Shedden R (2003) Long term records of riverine dissolved organic matter. Biogeochemistry 64:165–178
Yallop AR, Clutterbuck B, Thacker J (2010) Increases in humic dissolved organic carbon export from upland peat catchments: the role of temperature, declining sulphur deposition and changes in land management. Clim Res. doi:10.3354/cr00884
Acknowledgments
This research was supported by the U.S. Department of Energy’s Office of Science (BER) through the Midwestern Regional Center of the National Institute for Climatic Change Research at Michigan Technological University as well as the Michigan Technological University Ecosystem Science Center. We would like to thank Justina Silva and Jennifer Eikenberry for help with laboratory analysis, and Arvo Aljaste and Kristen Schmitt for help with field work. We appreciate the constructive comments from two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jan Mulder
Rights and permissions
About this article
Cite this article
Kane, E.S., Mazzoleni, L.R., Kratz, C.J. et al. Peat porewater dissolved organic carbon concentration and lability increase with warming: a field temperature manipulation experiment in a poor-fen. Biogeochemistry 119, 161–178 (2014). https://doi.org/10.1007/s10533-014-9955-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-014-9955-4