Abstract
Emergent vegetation in river beds can play a significant role in nutrient cycling in riverine sediments. We analysed and compared pore water NO3 − concentration gradients in the sediments of the River Leith, Cumbria, UK, in the presence and absence of emergent vegetation (dominated by Sparganium spp.). High resolution (1 cm interval), in situ, vertical profiles of NO3 − to 30 cm depth were measured using deployment of diffusive equilibrium in thin films probes on four occasions from July to September 2010. We found significantly (p < 0.05) lower NO3 − concentration under vegetated sediments (VS) compared to those under adjacent un-vegetated sediments (UVS). Concentrations of dissolved oxygen, measured in pore water collected from multi-level piezometers at 10, 20, 25, 30 and 35 cm depths at the VS and UVS sties, were generally lower under VS (median concentration = 28 μM) than under UVS (median = 132 μM) and correlated significantly with NO3 − concentration (Spearman’s r = 0.74, p < 0.05). Similarly, pore water dissolved organic carbon (DOC) concentration was 2.8 times higher under VS compared to UVS, and correlated negatively with NO3 − concentration (Spearman’s r = −0.39, p < 0.05). Specific ultra-violet absorption at 254 nm (SUVA) and per cent aromaticity values of DOC were significantly higher under VS (p < 0.05), suggesting that the DOC contained more complex (aromatic) compounds than DOC recorded under UVS. We suggest that, the higher quantity of DOC and its distinct SUVA and percentage aromaticity under VS may have supported faster dissolved oxygen consumption, with the creation of anoxic zones conducive for NO3 − reduction mainly through denitrification. Metabolic uptake and immobilization of NO3 − by plants and microbes may have further contributed to lower NO3 − concentrations under VS. As Sparganium spp. is a common plant growing in river beds in the UK, its role in NO3 − cycling should be considered in attempts to accurately budget N cycling in river beds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants influence nutrient cycling in soils and sediments directly through uptake of nutrients to meet metabolic demand and indirectly through priming microbial activities involved in nutrient transformations (Jackson et al. 2008). The indirect influence of plants on nutrient cycling involves the production of organic carbon through root exudates, sloughed-off cells and litter formation, which fuels diverse microbial processes, including N transformations (e.g. nitrification and denitrification) (Wigand et al. 2001; Jackson et al. 2008; Ullah and Moore 2009; Forshay and Dodson 2011). Consideration of plant impacts on nutrient cycling in the context of plant–microbe interaction in soils and sediments is critical for assessing the nutrient and pollution attenuation potential of ecosystems (Clarke 2002; Schneider and Melzer 2004; Jackson et al. 2008; Ullah and Moore 2009). Such processes are potentially important in riverine ecosystems, but as the vegetation growing in river beds tends to be patchy, its role in nutrient cycling is poorly studied (Sand-Jensen 1998; Groffman et al. 2005; Trimmer et al. 2009; Jackson et al. 2008; Forshay and Dodson 2011). Thus understanding the role of vegetation in influencing nutrient cycling is critical for accurate budgeting of nutrient cycling in the hyporheic zone (Groffman et al. 2005; Forshay and Dodson 2011).
According to Van Drecht et al. (2003), 10 % of the global riverine reactive N flux is derived from upwelling groundwater through riverine sediments into surface water. Nitrogen cascading into riverine sediments from upwelling groundwater, may be attenuated in the hyporheic zone through immobilization (sorption, and plant and microbial uptake) (Howard-Williams et al. 1982; Clarke 2002; Strauss and Lamberti 2002), and through reduction to N2 gas by denitrification before the reactive N joins river flows (Pretty et al. 2006; Krause et al. 2009; Trimmer et al. 2009; Stelzer et al. 2011; Forshay and Dodson 2011; Harrison et al. 2012). In addition to research looking at the influence of classic geomorphic features of rivers on nutrient cycling in riverine sediments, a number of researchers have also noted the importance of evaluating the influence of different vegetation types on nutrient cycling including those growing in riverbeds (Templer et al. 1996; Wigand et al. 2001; Clarke and Wharton 2001; Groffman et al. 2005; Trimmer et al. 2009; Lefebvre et al. 2006; Forshay and Dodson 2011).
Although the role of river bed vegetation on NO3 − attenuation in sediments is acknowledged, studies of NO3 − attenuation rates are restricted to surface sediments (ca. 3–10 cm below river bed) in the rooting zones of vegetation (Templer et al. 1996; Wigand et al. 2001; Groffman et al. 2005; Forshay and Dodson 2011). However, the stimulation of the activity of microbes, including those responsible for NO3 − attenuation, by vegetation may extend beyond typical rooting depths (from 5 to 12 cm) in the sediments: this possibility needs investigation to be able to account for sediment NO3 − attenuation more accurately in and beyond the rooting depth. Even though the aerial extent of vegetation growing in river beds could be small relative to the overall area of rivers, their role in mediating NO3 − attenuation in river bed sediment may be significant and hence worthy of investigation. This consideration is critical, particularly during base flow conditions in summer months (plant growth season), when the relative contribution of upwelling NO3 − rich groundwater to surface flows are high (Krause et al. 2009; Heppell et al. 2013).
Among vegetation types growing in riverbeds, emergent macrophytes dominated by Bur Reed (Sparganium erectum) is the most common species in UK rivers (O’Hare et al. 2010, 2012). O’Hare et al. (2010) reported the presence of this species in 47 rivers in a region spanning from southern Scotland to southern England. While the impact of this species on river flow modulation, Manning’s roughness co-efficient and trapping of fluvial sediments has been evaluated (Clarke 2002; Gurnell et al. 2006; O’Hare et al. 2012), to our knowledge, its influence on sediment nutrient attenuation, particularly NO3 −, has not been assessed.
The measurement and characterization of concentration gradients of solutes including NO3 − in river bed sediments has relied either on sediment core extraction from the riverbed with subsequent slicing at coarse depth intervals or pore water sampling from piezometers or multilevel samplers attached to piezometers (e.g. Krause et al. 2009). The suitability of these techniques is limited when the interest is in characterizing chemical gradients at a fine resolution (cm or less) to enable identification of ‘hot spots’ of biogeochemical activity taking place in micro-sites of sediments at cm or sub-centimetre scale (Köster et al. 2008). The capability of the diffusive equilibrium in thin films (DET) technique for measuring in situ NO3 − concentration gradient at high spatial resolution (cm at least) in riverbed sediments (Palmer-Felgate et al. 2010; Ullah et al. 2012) provides a means of resolving zones of NO3 − loss and gains due to biotic and/or abiotic processes (Dočekalová et al. 2002; Mortimer et al. 2002; Palmer-Felgate et al. 2010). Thus this technique allows capture of in situ fine scale dynamics of solutes including NO3 − (Dočekalová et al. 2002; Mortimer et al. 2002; Ullah et al. 2012). Recent research has deployed DET probes in riverine sediments for characterization of fine resolution profiles of NO3 −, NH4 + and soluble reactive phosphorus (Jarvie et al. 2008; Palmer-Felgate et al. 2010). The depth of DET deployment in these studies ranged from four to fifteen cm below the river bed. To our knowledge, there has been no published research on DET deployment to depths greater than 15 cm in river beds (Ullah et al. 2012), even though transformation of reactive N in river bed sediments is reported to be significant in deeper sediments (>15 cm depth) (Krause et al. 2009; Stelzer et al. 2011; Krause et al. 2013).
Here we use DET to evaluate the influence of emergent vegetation dominated by Sparganium spp. on NO3 − concentration gradients at high spatial resolution in riverbed sediments extending to 30 cm depth. We test the hypothesis that the NO3 − concentration gradient is lower in sediments under emergent vegetation than in bare sediments and explore possible mechanisms responsible for such phenomena. This study was part of a larger Natural Environment Research Council (NERC) project investigating the implications of river bed connectivity for nitrogen transformations in the hyporheic zone of a groundwater fed river in UK.
Materials and methods
Site description, DET deployment and pore water sampling
The study was undertaken in the River Leith (latitude 54°36′45″N; longitude 2°38′23″W), a groundwater-fed river in Cumbria, UK. The sediments in the experimental reach of the river were gravelly alluvium consisting of pebbles, gravels and sands (20–50 cm deep) overlying unconsolidated Penrith sandstone (Binley et al. 2013). Emergent vegetation in the riverbed grows in both the main channel and along the channel margins (at the interface of riparian and fluvial riverine eco-tones) and is dominated by Bur reed (S. erectum L) (O’Hare et al. 2012). The river meanders through a narrow floodplain and the catchment is predominantly agricultural with temporary grassland and some arable land use.
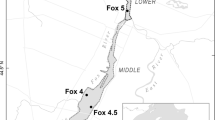
An emergent vegetated sediment patch (hereafter called ‘VS’) in the river channel dominated by Bur reed (S. erectum) and an adjacent un-vegetated riverbed sediment patch (hereafter called ‘UVS’) were selected for this study (Fig. 1). The study site for the research described in this paper lies within a 200 m reach of the River Leith used for the larger project: the detailed lithology and hydrology are given in Binley et al. (2013). For reference, Site C in Binley et al. (2013) corresponds to the UVS.
Particle size sieving of a sediment core collected from the thalweg of the river in the UVS revealed that about 50 % of the overlying sediments mainly consisted of gravels (>2 and <63 mm) in the upper 40 cm (Ullah et al. 2012; Binley et al. 2013), which is consistent with Krause et al. (2009). DET probes fabricated into 30 cm long plastic plates and protected in stainless steel holders were deployed into the river bed for four occasions between July and September 2010 coinciding with the main plant growth season. A single DET probe was deployed at each of three sites (C1, C2 and C3) to a depth of 30 cm into the river bed sediments in both the VS and UVS (Fig. 1). In the case of VS, the deployment of the DET probes on July 9, August 6 and September 10 was undertaken in the VS in the main river channel while the deployment on July 16 was undertaken in channel margin VS with relatively slow and/or sluggish flow conditions compared to the other three sites. Channel margin deployment of DET for NO3 profiles relative to NO3 − profiles at the UVS was undertaken at the same time to establish if emergent vegetation, irrespective of their location in the river, would have similar impacts on nitrate losses, as hypothesised in the introduction above. Details of the DET probe deployment techniques in River Leith are provided in Ullah et al. (2012). All deployments were undertaken under base flow conditions with discharges of 0.11, 0.27, 0.08 and 0.11 m3 s−1 on the day of DET probes retrieval in July to September. Typical base flow discharge in this river is around 0.1 m3 s−1 (Kaeser et al. 2009; Binley et al. 2013).
On each deployment date, the DET probe assemblies, secured within the stainless steel holders, was transferred on ice to the field and deployed to 30 cm depth next to piezometers in sites C1, C2 and C3 in the main channel VS and UVS. Once deployed, the DET probes were left in place for 70 h to ensure equilibration of NO3 − in the sediment pore water with the gel through diffusion (Mortimer et al. 1998). For anions and cations, a deployment time of 24–48 h for diffusion equilibration is recommended (Harper et al. 1997; Morford et al. 2003). We used at least 70 h to allow time for the disturbed sediments to collapse fully around the probe and re-establish conditions comparable to those prior to insertion of the DET assembly (Ullah et al. 2012). The rapid re-establishment of chemical gradients in sediments associated with redox conditions has already been recognised (Davison et al. 1994; Dočekalová et al. 2002). After 70 h, the deployed DET probes were retrieved, placed on ice and transferred to the laboratory within 1 h of retrieval. Prior to DET retrieval, pore water samples (25 mL) were collected from the multilevel samplers attached to the three piezometers at 10, 20 and 30 cm depth in the UVS (mid-channel) and at 10, 20, 25 and 35 cm depth in the VS in the August and September sampling. The samples were filtered on site through 0.45 μm cellulose acetate filters into borosilicate glass vials and transferred to the laboratory on ice with the DET probes. Piezometers, as part of the wider project, were not required to be installed in the channel margins, consequently head measurement and pore water sampling at this location was not possible at the time of DET retrieval.
Vertical hydraulic gradient and dissolved oxygen
Ground water head in six piezometers relative to the river stage was measured using a dip meter in the UVS and VS sites during the August and September DET deployment campaigns. The piezometer screen in the VS was at 30 cm depth while in the UVS it was at 20 cm depth in the gravelly alluvium. The difference in the groundwater level relative to the river stage (in cm) was used to calculate vertical hydraulic gradient (VHG) as:
where d h is difference in elevation of water level in the piezometer relative to the stream stage and d l is the distance from the river bed to the mid-screen of the piezometer (Kalbus et al. 2006). Based on our calculations, a positive VHG is representative of groundwater upwelling and negative VHG indicates surface water downwelling potentials (Binley et al. 2013).
For the measurement of dissolved oxygen content during the August and September campaigns, pore water samples were collected from the multilevel samplers (5 mL, as above). Pore water was transferred slowly from the collection syringe via a three-way stopcock into an open syringe barrel containing a calibrated, fast response dissolved oxygen electrode. The 50 μm electrode had a stainless steel protective guard, and was connected via an inline amplifier to a data-logging meter (Under Water-Meter, Unisense, Denmark). The instrumental detection limit for dissolved oxygen was 3 μM with a precision of ~2 %.
Laboratory processing of DET
The DET probes were processed in the laboratory immediately on arrival from the field site. The whole assembly of the stainless steel holder was placed on a bench top and the gel with the filter membrane was cut out from the window using a Teflon coated razor blade. The filter membrane over the DET gel was carefully removed and the gel was transferred to a glass board. The gel was then cut at 1 cm intervals with the razor blade within 2 h of its retrieval. Slicing within this time avoids significant peak relaxation of anions and cations beyond 1 cm intervals through lateral diffusion in the gel (Harper et al. 1997). Gel sections damaged during deployment and/or retrieval from the river bed, were discarded from further analysis. The sectioned gel strips were transferred into pre-labelled 1.5 mL vials. High purity water (Milli Q, Millipore), referred to here as MQ water was added to each vial containing the gel. Addition of 1,000 μL of MQ water was equal to 6 times dilution of the NO3 − concentration in the gel. Previous mock deployment of DET in June 2012, showed that 6–12 times dilution of NO3 − in 1 cm gel slice through back equilibration allowed measurement above the limit of detection (LOD) of the ion chromatograph (DIONEX) used for NO3 − determination. The vials containing DET gel were capped, placed on frozen ice packs and shaken overnight on a reciprocating shaker to allow back equilibration of NO3 − in the gel with the MQ water. Next day, the NO3 − extract from the gel was analysed using DIONEX. At least ten blanks were run with the suite of analysis from each deployment for the determination of LOD. The LOD for NO3 − was 0.02 mg N L−1 with an analytical precision of ±6.6 %.
Processing and analysis of pore water samples
The pore water samples were analysed for dissolved organic carbon (DOC) content using a TOC analyser (Thermalox TOC-TN analyser). The TOC analyser purged the acidified samples before analysis for the determination of non-purgeable DOC contents. The LOD of DOC was 0.32 and 0.40 mg C L−1 with analytical precision (repeat quality control standards) of 4.8 and 1.3 % for the August and September campaign samples, respectively.
Pore water samples were also scanned on a UV–Vis spectrophotometer in a 1 cm quartz window cuvette. MQ water was used for blank correction of the scan. The absorbance at 250 and 280 nm was recorded for the determination of SUVA@254nm(specific UV absorption (L mg−1 C m−1) = (A254nm × 100)/DOC) and percent aromaticity (% aromaticity = [0.05 (A280nm × 1,000) + 6.74)] (Chin et al. 1994; Roehm et al. 2009; Kothawala et al. 2012).
Sediment sampling and analysis
An intact sediment core (30 cm long) was collected from each DET deployment site for both UVS and VS (main channel and channel margin) in September 2010 (Fig. 1). A petrol driven Cobra TT drill was used to drill the river bed using a 50 mm corer equipped with a plastic liner as in Binley et al. (2013). The collected cores were sectioned into 5 cm interval at the field site, placed in zip-lock bags, transferred to the laboratory on ice and frozen until further analysis.
The sediment sections were homogenized and sieved (2 mm sieve). The sediment fraction <2 mm in size was then used for the extraction of DOC and NH4 + content and gravimetric water contents. A sub-sample of 10 g dry equivalent was weighed into a 250 mL plastic bottle. Ammonia was extracted by adding 50 mL of 2 M KCl solution shaking gently for 1 h and filtering through 0.45 μm filter. The filtrate was analysed within 24 h of extraction for the determination of NH4 + using a discrete colorimetric (SEAL AQ2) analyser. The LOD for NH4 + was 0.003 mg N L−1 with an analytical precision of 3.2 %. The extraction procedure for DOC was similar to that for NH4 +, except 50 mL MQ water was used for extraction instead of the KCl salt. Extracted DOC was filtered and analysed on a TOC-TN analyser (Thermalox analyser) as above.
Statistical analysis
Dissolved oxygen and DOC measurements from all depths (10, 20, 25, and 30 cm depths) below the river bed and all sites were pooled together for correlation analysis with NO3 − measured using DET at corresponding depths to that of the multilevel samplers. As the data were not normally distributed, non-parametric Spearman’s correlations were determined. The mean NO3 − concentration of each deployment campaign (mean of three profiles) was tested for significant difference between UVS and VS using the non-parametric Mann–Whitney U test at 5 % significance level. SUVA and aromaticity of DOC was normally distributed (Shapiro–Wilk test, p > 0.5) under UVS and VS were tested for differences using t test at 5 % significance level.
Results
When all data from the four deployments were pooled together, the concentration of NO3 − ranged from 0.41 to 9.83 mg NO3 −−N L−1 under UVS (median value = 4.28 mg N L−1), while in the VS it ranged from 0.05 to 7.31 mg NO3 −−N L−1 (median value = 0.50 mg N L−1). Average concentration of NO3 − in the surface water of the river measured at the time of DET retrievals was 1.71 mg N L−1. NO3 − profiles showed a significant difference (p < 0.05) between UVS and VS over the four deployment campaigns (Fig. 2) except at Site C1 in September. Generally, VS both in the main river and along the channel margins had a similar relationship with NO3 − concentration gradients compared to profiles of NO3 − under UVS (Fig. 2). Fine resolution NO3 − profiles under the VS showed a decreasing trend in concentration in surface sediments from 1 to ~10 cm depth except in Site C2 and C2 of the channel margin VS. The median concentration of NO3 − under VS at 1 cm depth was 1.85, declining to 1.44 at 5 cm, and to 0.37 mg N L−1 at 10 cm depth. This trend represented a fivefold decrease in median NO3 − concentration at 10 cm compared to concentration at 1 cm depth below the river bed under VS than under UVS.
NO3 − profiles of vegetated and un-vegetated river bed sediments measured through DET. Missing NO3 − data at a specific depth (s) in these profiles represent a damaged DET gel section (during deployment and/or retrieval from the river bed) that was discarded from analysis. Note that NO3 − profiles of July 16 represent a comparison of the channel margin vegetated sediments to the un-vegetated sediments, while the rest represent vegetated sediments located in the main channel
Dissolved oxygen concentrations in the pore water showed variability with depth in the UVS and VS; however, the concentrations in the VS were generally lower than in the UVS sediments (Fig. 3). Dissolved oxygen concentrations ranged from 39 to 200 μM (median = 136 μM) in the UVS and from <LOD to 199 μM (median = 42 μM) in the VS. Dissolved oxygen exhibited significant positive correlation with NO3 − concentration (Spearman’s r = 0.74, p < 0.05, n = 29). Dissolved oxygen in the surface water of the river measured at the time of DET retrievals was 572 and 369 μM in August and September, respectively.
Dissolved oxygen concentration (in μM) measured in the sediment pore water at 10, 20, and 30 cm depth in the un-vegetated and at 10, 20, 25, and 35 cm depths in the vegetated river sediments. Note that the dissolved oxygen at 25 cm depth under the vegetated sediments in site C2 measured in August 2010 was lower than the instrumental detection limit for dissolved oxygen (3 μM)
Pore water DOC values varied with depth and ranged from 0.95 to 12.90 mg C L−1 in the UVS (median = 2.05 mg C L−1) and from 1.86 to 16.60 mg L−1 in the VS (median = 5.73 mg C L−1) (Fig. 4). Pore water DOC values showed a significant negative correlation with NO3 − concentration (Spearman’s r = −0.39, p < 0.05, n = 30). DOC extracted from the sediments were also relatively higher in VS in the top 0–10 cm depths than in the UVS (Fig. 5a); however, the differences between VS and UVS were not statistically significant (at 5 % significant level) due to the high variability of DOC concentrations. Similar to the extracted DOC, mean NH4 + content in the sediment was also relatively higher under VS in the top 0–10 cm depths relative to the UVS (Fig. 5b) although the difference was not significant statistically.
Specific UV absorption of the pore water DOC samples in all depths ranged from 0.18 to 1.76 L mg−1 C m−1in the UVS (mean = 0.72) and from 0.31 to 2.29 L mg−1 C m−1 in the VS (mean = 1.33) (Table 1). Similarly, the aromaticity of these samples ranged from 6.76 to 8.22 % in the UVS (mean = 7.13 %) and from 7.28 to 9.36 % (mean = 8.23 %) in the VS. SUVA measurement based on absorption at 254 nm correlated significantly (R2 = 0.77, p < 0.05) with aromaticity measurement based on absorption at 280 nm. Based on two sample t test, mean SUVA and aromaticity of pore water samples collected from VS were significantly higher than those collected from UVS (p = 0.02, and p = < 0.001, respectively). Vertical hydraulic gradient at 20 cm depth in the UVS averaged 16.2 and 16.7 %, while in the VS (at 30 cm depth) it averaged 2.8 and 4.4 % in August and September, respectively, indicating upwelling potentials at both sites.
Discussion
There were marked differences in the NO3 − concentration gradients between VS and UVS under this groundwater fed stretch of River Leith (Binley et al. 2013). We did not directly measure NO3 − reduction processes (e.g. denitrification) or NO3 − uptake by plants and immobilization by microbes under the VS; however, the observed trends in NO3 − concentration and its significant correlation with dissolved oxygen and DOC indicate that riverine sediments under emergent vegetation may reflect a micro-environment conducive to faster NO3 − loss, resulting in lower pore water NO3 − concentrations compared to UVS. The significant difference in NO3 − concentration between UVS and VS was observed consistently over the measurement period (July to September). Wigand et al. (2001) reported significantly lower pore water nutrient concentrations in river bed sediments planted with macrophytes compared to bare sediments. Similarly, Forshay and Dodson (2011) observed significantly faster NO3 − reduction through denitrification under vegetated riverine sediments compared to bare sediments, which signifies the benefit of riverine vegetation in stimulating NO3 − reduction and improving water quality. Other than reduction through denitrification, biotic uptake of NO3 − by plants during the growing season could have been substantial. Harrison et al. (2012) observed NO3 − uptake by plants from 42 to 63 % during the plant growth season in relic oxbow-wetlands dominated by emergent vegetation. Dissimilatory nitrate reduction to ammonium (DNRA) could be an additional sink for pore water nitrate loss beneath VS (Kelso et al. 1997); however, the potential for NO3 − loss through DNRA was of minimal importance compared to denitrification in the un-vegetated sediments of River Leith (Lansdown et al. 2012). That said, dilution of pore water NO3 − by down-welling surface water (with lower NO3 − levels than the upwelling groundwater) may have been another possibility for the observed low NO3 − under VS; however, the likelihood of significant dilution effect particularly in sediments deeper than 10 cm appear minor given the fact that the river is predominantly groundwater-fed under base flow conditions (Kaeser et al. 2009; Binley et al. 2013). Fine sediments in the surface water trapped by vegetation may have led to lower VHG % (2.8–4.4 %) beneath the VS with potential for relatively longer pore water residence time than under the UVS (VHG of 16.2–16.7 %). Longer pore water residence times under VS may have been an additional factor causing enhanced losses of NO3 − under VS (Krause et al. 2013). However, such an influence appears minimal relative to direct plant mediated losses (nitrate reduction and plant uptake), given the fact that another un-vegetated pool site (~7 m upstream of the VS) also had a similar range of VHG (2.5–7.5 %) to that of the VS, yet its median NO3 − in the upper 30 cm depth was 6.33 mg N L−1 (data not shown) compared to 0.50 mg NO3 −−N L−1 beneath the VS.
The difference in NO3 − profiles between UVS and VS was evident up to 30 cm depth, which is beyond the rooting zone of emergent vegetation (Sparganium spp.) in the River Leith (~5–10 cm). Our data suggest that the presence of vegetation may have promoted NO3 − reduction in bed sediments that are deeper than the rooting zone. Significant down welling of surface water into the river bed sediments during high flow events have been reported by Byrne et al. (2013) in this river, which in case of the vegetated sediments, may be responsible for the delivery of DOC into sediment pore water deeper than the typical rooting depth. This observation implies that it is important to consider the impact of vegetation in deeper sediments beyond the rooting zone for accurate quantification of NO3 − attenuation mediated by vegetation.
Lithologically, the experimental reach of the River Leith at ~100 cm depth consists of unconsolidated sandstone bedrock overlain by gravelly alluvium (Binley et al. 2013). Pore water NO3 − concentration measured at three occasions at 100 cm depth in the sandstone bedrock in July, August and September of 2009 and 2010, downstream (in the UVS) and upstream of the VS averaged at 5.36 ± 1.45 mg N L−1 (mean ± standard deviation) (data not shown) and the concentrations ranged from 1.03 to 7.21 mg N L−1. Other than the UVS at the downstream site, the upstream site was also un-vegetated and was located about 7 m upstream of the VS. Krause et al. (2009) reported similar NO3 − concentrations in the sandstone bedrock in this river (ranging from 3.5 to 6.6 mg N L−1). We did not measure NO3 − concentration in the VS at 100 cm depth, but, given the consistent distribution of sandstone bedrock underlying the gravelly alluvium in this river (Binley et al. 2013), we assume a similar likely range in NO3 − concentration at 100 cm depth under the VS. As the mean NO3 − concentration in the upper 30 cm in the VS was much lower (mean = 0.53 mg N L−1), it is very likely that the key driver of NO3 − loss in the upwelling water in the upper 30 cm depth in the VS are NO3 − reduction and loss processes enhanced by the presence of vegetation. Moreover, the influence of vegetation on sediment pore water NO3 − concentration was similar, irrespective of whether the vegetation was growing in the main channel or along the channel margins. The channel margin site was downstream of the UVS and next to another instrumented un-vegetated site, where the mean NO3 − concentration was 2.7 mg N L−1 (data not shown) in the upper 30 cm compared to the 0.62 mg N L−1 beneath the channel margin VS. This observation supports our contention that emergent vegetation mainly mediated NO3 − losses. Mean sediment extracted DOC in the upper 10 cm in the channel margin sediments was also relatively higher than the DOC in the UVS (135 and 46 μg C g−1 sediment, respectively), which may have promoted faster NO3 − losses, similar to those observed in the mid-channel VS.
The median concentration of dissolved oxygen in the upper 30 cm of the VS was 202 % lower than in the UVS. Lower dissolved oxygen concentration in the VS may have promoted greater loss of NO3 − through denitrification in the upwelling groundwater compared to UVS. In general, NO3 − concentrations were lower in locations within the sediments where dissolved oxygen contents were low, and consequently there was a positive correlation between NO3 − and dissolved oxygen. NO3 − reduction and loss in marine and riverine sediments have been reported at dissolved oxygen concentration of ~<90 μM (Dalsgaard and Revsbech 1992; Olde Venternik et al. 2003; Pretty et al. 2006). Similarly, Tessoriero and Puckett (2011) also observed faster NO3 − reduction through denitrification when dissolved oxygen concentrations were <60 μM in shallow aquifers. When the dissolved oxygen data was pooled to calculate the percentile distribution of the data range (box plot not shown), the concentration of dissolved oxygen in pore water in the VS at the 87th percentile was less than 90 μM compared to UVS where concentration <90 μM were at the 33rd percentile. This pattern of dissolved oxygen in our study suggests that VS may induce extensive reduced conditions in sediments favourable for NO3 − reduction due to respiration of DOC exudates relative to the UVS. Although aquatic vegetation transport oxygen through the aerenchyma tissues into bed sediments (Caraco et al. 2006), the observed low concentration of dissolved oxygen under the VS may be indicative of relatively faster consumption than possible replenishment through plant root transport into the sediments (Calhoun and King 1997).
Even though weak, a significant negative correlation was observed between DOC and NO3 − suggesting that sediments with high DOC contents may support elevated NO3 − reduction. This is consistent with the observations of Lansdown et al. (2012), who reported that NO3 − reduction was limited by the availability of dissolved organic carbon in this river. Median pore water DOC concentrations over the 30 cm depth in VS were 186 % greater than DOC under UVS. This difference in DOC may have been due to root exudates and decomposition of dead plant material contributed by the emergent vegetation. Moreover, emergent and submerged macrophytes in river beds have also been found more effective than bare sediment patches in trapping fine sediments from fluvial sources (Heppell et al. 2009) and exogenous particulate organic matter (Sand-Jensen 1998; Trimmer et al. 2009; O’Hare et al. 2012). The trapped fine sediments with particulate organic matter could have been an additional source of the relatively higher DOC delivery into the underlying pore water through surface water down welling during high flow events (Gu et al. 2008; Byrne et al. 2013). S. erectum patches across 47 river reaches in the UK contained 1.8 times higher organic matter than bare river bed sediments (O’Hare et al. 2012), which indicates higher potential for dissolved oxygen consumption through microbial respiration (Sobczak and Findlay 2002; Trimmer et al. 2009) and release of DOC to serve as an electron donor during NO3 − reduction (Forshay and Dodson 2011).
The probable mix of autochthonous and allochthonous sources of DOC in sediment pore water in the VS appear to have led to distinct SUVA and aromaticity values compared to DOC in the UVS. Markedly higher SUVA and aromaticity of pore water DOC in the VS probably reflect a mix of DOC molecules containing more aromatic compounds than the DOC in the UVS (Chin et al. 1994; Weishaar et al. 2003; Kothawala et al. 2012). Given the difference in spectrophotometric properties between DOC in the VS and UVS, the reactivity and bioavailability of the DOC could have differed between the two sediment types. Some authors have speculated that DOC in vegetated riverine sediments is more complex (Strauss and Lamberti 2002; Findlay 2003) than the DOC in UVS, which is in agreement with our findings. Lansdown et al. (2012) found enhanced potential denitrification rates in response to addition of a complex organic C substrate (bacteriological peptone) to sediment slurries compared to un-amended slurries collected from un-vegetated sediments of this river. Therefore, it appears that DOC of higher quantity and of a complex nature beneath the VS may have supported faster NO3 − reduction (Strauss and Lamberti 2002; Findlay 2003; Forshay and Dodson 2011). The observation of high pore water DOC and low NO3 − beneath VS suggests that NO3 − reduction may be NO3 − rather than DOC limited under anoxic conditions in VS, while DOC limited in UVS (Lansdown et al. 2012). This observation is in agreement with those of Forshay and Dodson (2011) who concluded that denitrification in riverine vegetated sediments was NO3 − limited. Furthermore, both SUVA and aromaticity also correlated significantly with NO3 − (Spearman’s r = −0.52 and −0.72, respectively, with p < 0.05, n = 20). Since the lability of the DOC in supporting oxygen consuming heterotrophs and denitrification in the VS was not evaluated directly, we recommend further studies to elucidate if DOC of a complex aromatic nature is supportive of denitrifier and general heterotrophic microbial activity.
DOC and NH4 + extracted from the sediments were generally higher at 0–10 cm and for the VS there was a sharp decline in concentration from 10 to 30 cm depth (Fig. 5). A fivefold decrease in NO3 − concentration measured using DET between 1 and 10 cm depth (Fig. 2) supports the contention that high sediment and pore water DOC in the VS may have supported NO3 − reduction (Groffman et al. 2005; Forshay and Dodson 2011; Borin and Salvato 2012). Median dissolved oxygen concentration over the measurement period also decreased from 70 μM at 10 cm depth to 25 μM at 20 cm depth in the VS, with no such consistent trend evident in the UVS (Fig. 3). Thus sediments beneath vegetation are expected to be highly reactive biogeochemically (Forshay and Dodson 2011), and can exert strong control on NO3 − losses beyond the rooting depth within permeable sediments. This was evident from the consistently lower NO3 − concentration gradient (from about 10 to 30 cm depth) in the VS, except in September in Site C2 where concentration peaks at 14 and 26 cm depths were followed by decreasing NO3 −. A detailed geochemical interpretation of these concentration peaks measured through DET is desirable with respect to coupled nitrification–denitrification in sediments (Caffrey and Kemp 1992; Risgaard-Petersen and Jensen 1997), but is beyond the scope of the available data. Nevertheless, high resolution NO3 − profiles measured using DET, which could not be obtained using coarse-scale multilevel samplers (Ullah et al. 2012) offer the potential for resolving zones of losses and gains in armoured riverine sediments due to physical and biogeochemical processes in future studies. The profiles of NO3 − in the UVS also exhibited distinct peaks, particularly in site C2 in August and in sites C1, C2 and C3 in September. These localized zones of gain in the concentration of NO3 − in the UVS appear to be driven by production processes of NO3 − (nitrification), in agreement with similar observation of gains in NO3 − concentration along the upwelling flow paths in the same experimental stretch of the River Leith as reported in Krause et al. (2009).
Conclusions
This study provides evidence for enhanced NO3 − attenuation in riverine sediments under emergent vegetation compared to adjacent UVS in a predominantly upwelling reach of the River Leith. The pore water NO3 − concentration gradient in the sediment under emergent vegetation growing in the main channel and along channel margins was lower than that observed in UVS. This difference was not limited to the plant rooting zone but was recorded beyond this zone up to 30 cm depth using a specially adapted DET sampling system (Ullah et al. 2012). We suggest that the emergent vegetation may act as a source of higher and structurally more complex DOC concentrations in the sediment pore water relative to UVS and the vegetation may have mediated the consumption of dissolved oxygen (through root and microbial respiration) with consequent provision of conducive conditions for NO3 − reduction. Metabolic uptake by plants and microbes may have been additional temporary biotic sinks for NO3 − in the VS compared to the UVS. The overall role of emergent vegetation, particularly Sparganium spp., in NO3 − attenuation need consideration while evaluating options for nutrient management and restoration of biogeochemical functions rendered by riverine ecosystems. Further studies on quantifying plant mediated NO3 − reduction processes in riverine sediments, particularly denitrification relative to plants uptake, both during growing and non-growing seasons, are recommended (Borin and Salvato 2012; Harrison et al. 2012).
References
Binley A, Ullah S, Heathwaite AL, Heppell CM, Byrne P, Lansdown K, Trimmer M, Zhang H (2013) Revealing the spatial variability of water fluxes at the groundwater-surface water interface. Water Resour Res 49:1–15
Borin M, Salvato M (2012) Effects of five macrophytes on nitrogen remediation and mass balance in wetland mesocosm. Ecol Eng (in press)
Byrne P, Binley A, Heathwaite AL, Ullah S, Heppell CM, Lansdown K, Zhang H, Trimmer M, Keenan P (2013) Control of river stage on the reactive chemistry of the hyporheic zone. Hydrol Process (in press)
Caffrey JM, Kemp WM (1992) Influence of the submerged plant, Potamogeton perfoliatus, on nitrogen cycling in estuarine sediments. Limnol Oceanogr 37:1483–1495
Calhoun A, King GM (1997) Regulation of root associated methanotrophy by oxygen availability in the rhizosphere of two aquatic macrophytes. Appl Environ Microbiol 63:3051–3058
Caraco N, Cole J, Findlay S, Wigand C (2006) Vascular plants as engineers of oxygen in aquatic systems. Bioscience 56:219–225
Chin Y, Alken G, O’Loughlin E (1994) Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ Sci Technol 28:1853–1858
Clarke SJ (2002) Vegetation growth in rivers: influences upon sediment and nutrient dynamics. Prog Phys Geogr 26:159–172
Clarke SJ, Wharton G (2001) Sediment nutrient characteristics and aquatic macrophytes in lowland English rivers. Sci Tot Environ 266:103–112
Dalsgaard T, Revsbech NP (1992) Regulating factors in trickling filter biofilms as measured with the oxygen/nitrous oxide microsensor. FEMS Microbiol Ecol 101:151–164
Davison W, Zhang H, Grime GW (1994) Performance characteristics of gel probes used for measuring the chemistry of pore water. Environ Sci Technol 28:1623–1632
Dočekalová H, Clarisse O, Salmomon S, Wartel M (2002) Use of constrained DET probe for a high resolution determination of metals and anions distribution in the sediment pore water. Talanta 51:145–155
Findlay S (2003) Bacterial response to variation in dissolved organic matter, In: Findlay S, Sinsabaugh RL (eds) Aquatic ecosystems: interactivity of dissolved organic matter. Academic Press, Punta Gorda, p 363–379
Forshay KJ, Dodson SI (2011) Macrophyte presence is an indicator of enhanced denitrification and nitrification in sediments of a temperate restored agricultural streams. Hydrobiologia 668:21–34
Groffman PM, Dorsey AM, Mayer PM (2005) N processing within geomorphic structures in urban streams. J N Am Benthol Soc 24:613–625
Gu C, Hornberger GM, Herman JS, Mills AL (2008) Influence of stream-groundwater interactions in the streambed sediments on NO3 − flux to a low-relief coastal stream. Water Resour Res 44:W11432. doi:10.1029/2007WR006739
Gurnell AM, Van Oosterhout MP, De Vlieger B, Goodson JM (2006) Reach scale interactions between aquatic plants and physical habitat: river Frome, Dorset. River Res Appl 22:667–680
Harper MP, Davison W, Zhang H, Tych W (1997) Temporal, spatial and resolution constraints for in situ sampling devices using diffusional equilibration: dialysis and DET. Environ Sci Technol 31:3110–3119
Harrison MD, Groffman PM, Mayer PM, Kaushal S (2012) Nitrate removal in two relict oxbow urban wetlands: a 15 N mass-balance approach. Biogeochemistry 111:647–660
Heppell CM, Wharton G, Cotton JAC, Bass JAB, Roberts SE (2009) Sediment storage in the shallow hyporheic of lowland vegetated river reaches. Hydrol Process 23:2239–2251
Heppell CM, Heathwaite AL, Binley A, Byrne P, Ullah S, Lansdown K, Keenan P, Trimmer M, Zhang H (2013) Interpreting spatial patterns in redox and coupled water-nitrogen fluxes in the streambed of a gaining river reach. Biogeochemistry (in press)
Howard-Williams C, Davies J, Pickmere S (1982) They dynamics of growth, the effects of changing area and nitrate uptake by watercress Nasturtium officinale R. Br. in a New Zealand stream. J Appl Ecol 19:589–601
Jackson LE, Burger M, Cavagnaro TR (2008) Roots, nitrogen transformations and ecosystem services. Annu Rev Plant Biol 59:341–363
Jarvie HP, Mortimer RJG, Palmer-Felgate EJ, Quinton KS, Harman SA, Carbo P (2008) Measurement of soluble reactive phosphorus concentration profiles and fluxes in river-bed sediments using DET gel probes. J Hydrol 350:261–273
Kaeser DH, Binley A, Heathwaite AL, Krause S (2009) Spatio-temporal variations of hyporheic flow in a riffle-step-pool sequence. Hydrol Process 23:2138–2149
Kalbus E, Reinstorf F, Schirmer M (2006) Measuring methods for groundwater-surface water interactions: a review. Hydrol Earth Syst Sci 10:873–887
Kelso BHL, Smith RV, Laughlin RJ, Lennox SD (1997) Dissimilatory nitrate reduction in anaerobic sediments leading to river nitrite accumulation. Appl Environ Microbiol 63:4679–4685
Köster M, Wardenga R, Blume M (2008) Microscale investigation of microbial communities in coastal surficial sediments. Mar Ecol 29:89–109
Kothawala DN, Roehm R, Blodau C, Moore TR (2012) Selective adsorption of dissolved organic matter to mineral soils. Geoderm 189(190):334–342
Krause S, Heathwaite AL, Binley A, Keenan P (2009) Nitrate concentration changes at the groundwater-surface water interface of a small Cumbrian river. Hydrol Process 23:2195–2211
Krause S, Ticklenburg C, Munz M, Naden M (2013) Streambed nitrogen cycling beyond the hyporheic zone: flow controls on horizontal patterns and depth distribution of nitrate and dissolved oxygen in the up-welling groundwater of a lowland river. JGR-Biogeosci. doi:10.1029/2012JG002122
Lansdown K, Trimmer M, Heppell CM, Sgouridis F, Ullah S, Heathwaite AL, Binley A, Zhang H (2012) Characterization of the key pathways of dissimilatory nitrate reduction and their response to complex organic substrates in hyporheic sediments. Limnol Oceanogr 57(2):387–400. doi:10.4319/lo.2012.57.2.0387
Lefebvre S, Marmonier P, Peiry JL (2006) Nitrogen dynamics in rural streams: differences between geomorphologic units. Ann Limnolo-Int J Lim 42:43–52
Morford J, Kalnejais L, Martin W, Francoois R, Karle IM (2003) Sampling marine porewater for Mn, Fe, U, Re and Mo: modification of diffusional equilibration think film gel probes. J Exp Marine Biol Ecol 285:85–103
Mortimer RJG, Krom MD, Hall POJ, Hulth S, Stahl H (1998) Use of gel probes for the determination of high resolution solute distribution in marine and estuarine pore water. Mar Chem 63:119–129
Mortimer RJG, Krom MD, Harris SJ, Hayes PJ, Davies IM, Davison W, Zhang H (2002) Evidence for suboxic nitrification in recent marine sediments. Mar Ecol Prog Ser 236:31–35
O’Hare JM, McGahey C, Bissett N, Cailes C, Henville P, Scarlet P (2010) Variability in roughness measurement for vegetated rivers near base flow, in England and Scotland. J Hydrol 385:361–370
O’Hare JM, O’Hare MT, Gurnell AM, Scarlett PM, Liffen T, McDonald C (2012) Influence of an ecosystem engineer, the emergent macrophyte Sparganium erectum, on seed trapping in lowland rivers and consequences for landform colonization. Freshw Biol 57:104–115
Olde Venternik H, Hummelink E, Van Den Hoorn MW (2003) Denitrification potential of a river floodplain during flooding with nitrate-rich water: grasslands versus reedbeds. Biogeochemistry 65:233–244
Palmer-Felgate EJ, Mortimer RJ, Krom MD, Jarvie HP (2010) Impact of point-source pollution on phosphorus and nitrogen cycling in stream-bed sediments. Environ Sci Technol 44:908–914
Pretty JL, Hildrew AG, Trimmer M (2006) Nutrient dynamics in relation to surface-subsurface hydrological exchange in a groundwater fed chalk stream. J Hydrol 330:84–100
Risgaard-Petersen N, Jensen K (1997) Nitrification and denitrification in the rhizosphere of the aquatic macrophyte Lobelia dotmanna. L Limnol Oceanogr 42:529–537
Roehm CL, Giesler R, Karlsson J (2009) Bioavailability of terrestrial organic carbon to lake bacteria: the case of a degrading sub-arctic permafrost mire complex. J Geophys Res 114:G030006. doi:10.1029/2008JG000863
Sand-Jensen K (1998) Influence of submerged macrophytes on sediment composition and near-bed flow in lowland streams. Freshw Biol 39:663–679
Schneider S, Melzer A (2004) Sediment and water nutrient characteristics in patches of submerged macrophytes in running waters. Hydrobiologia 527:195–207
Sobczak WV, Findlay S (2002) Variation in bioavailability of dissolved organic carbon among stream hyporheic flowpaths. Ecology 83:3194–3209
Stelzer RS, Bartsch LA, Richardson WB, Srauss EA (2011) The dark side of the hyporheic zone: depth profiles of nitrogen and its processing in stream sediments. Freshw Biol 56:2021–2033
Strauss EA, Lamberti GA (2002) Effect of dissolved organic carbon quality on microbial decomposition and nitrification rates in stream sediments. Freshw Biol 47:65–74
Templer P, Findlay S, Wigand C (1996) Sediment chemistry associated with native and non-native emergent macrophytes of the Hudson River marsh ecosystem. Section II. In: Waldman JR, Nieder WC, Blair EA (eds) Final reports of the Tibor T. Polgar Fellowship Program, 1995. Hudson River Foundation, New York, p 32
Tessoriero AJ, Puckett LJ (2011) O2 reducation and denitrification rates in shallow aquifers. Water Resour Res 47:W12522. doi:10.1029/2011WR010471
Trimmer M, Sanders IA, Heppell CM (2009) Carbon and nitrogen cycling in a vegetated lowland chalk river impacted by sediment. Hydrol Process 23:2225–2238
Ullah S, Moore TR (2009) Soil drainage and vegetation controls of nitrogen transformation rates in forest soils, southern Quebec. J Geophys Res 114:G01014. doi:10.1029/2008JG000824
Ullah S, Zhang H, Heathwait AL, Binley A, Lansdown K, Heppell CM, Trimmer M (2012) In situ measurement of redox sensitive solutes at high spatial resolution in a riverbed using diffusive equilibrium in thin films (DET). Ecol Eng 49:18–26
Van Drecht G, Bouwman AF, Knoop JM, Beusen AHW, Meinardi CR (2003) Global modelling of the fate of nitrogen from point and non-point sources in the soils, groundwater, and surface water. Global Biogeochem Cycles 17:1115
Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37:4702–4708
Wigand C, Finn M, Findlay S, Fischer D (2001) Submerged macrophyte effects on nutrient exchanges in riverine sediments. Estuaries 24:398–406
Acknowledgments
We thank Paddy Keenan, Neil Mullinger, Hao Cheng and Muhammad Al-Shafie for their help in field work and sample processing in the Centre for Sustainable Water Management nutrient laboratories at Lancaster University. We thank Lowther Estate in Cumbira for allowing us access to the site and Patrick Byrne at Liverpool John Moors University for his help in the production of the site map. The research was supported by Natural Environment Research Council Grant NE/F006063/1 awarded to Lancaster University and Queen Mary, University of London (NE/F004753/1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: James Sickman
Rights and permissions
About this article
Cite this article
Ullah, S., Zhang, H., Heathwaite, A.L. et al. Influence of emergent vegetation on nitrate cycling in sediments of a groundwater-fed river. Biogeochemistry 118, 121–134 (2014). https://doi.org/10.1007/s10533-013-9909-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-013-9909-2