Abstract
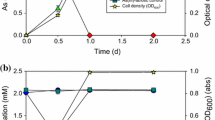
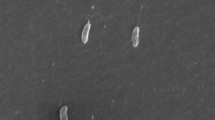
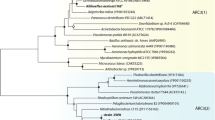
Arsenic is a carcinogenic compound widely distributed in the groundwater around the world. The fate of arsenic in groundwater depends on the activity of microorganisms either by oxidizing arsenite (AsIII), or by reducing arsenate (AsV). Because of the higher toxicity and mobility of AsIII compared to AsV, microbial-catalyzed oxidation of AsIII to AsV can lower the environmental impact of arsenic. Although aerobic AsIII-oxidizing bacteria are well known, anoxic oxidation of AsIII with nitrate as electron acceptor has also been shown to occur. In this study, three AsIII-oxidizing bacterial strains, Azoarcus sp. strain EC1-pb1, Azoarcus sp. strain EC3-pb1 and Diaphorobacter sp. strain MC-pb1, have been characterized. Each strain was tested for its ability to oxidize AsIII with four different electron acceptors, nitrate, nitrite, chlorate and oxygen. Complete AsIII oxidation was achieved with both nitrate and oxygen, demonstrating the novel ability of these bacterial strains to oxidize AsIII in either anoxic or aerobic conditions. Nitrate was only reduced to nitrite. Different electron donors were used to study their suitability in supporting nitrate reduction. Hydrogen and acetate were readily utilized by all the cultures. The flexibility of these AsIII-oxidizing bacteria to use oxygen and nitrate to oxidize AsIII as well as organic and inorganic substrates as alternative electron donors explains their presence in non-arsenic-contaminated environments. The findings suggest that at least some AsIII-oxidizing bacteria are flexible with respect to electron-acceptors and electron-donors and that they are potentially widespread in low arsenic concentration environments.




Similar content being viewed by others
References
Ahn SJ, Costa J, Emanuel JR (1996) PicoGreen quantitation of DNA: effective evaluation of samples pre- or post-PCR. Nucleic Acids Res 24(16):2623–2625
Ascar L, Ahumada I, Richter P (2008) Influence of redox potential (Eh) on the availability of arsenic species in soils and soils amended with biosolid. Chemosphere 72(10):1548–1552
Beauchemin S, Kwong YTJ (2006) Impact of redox conditions on arsenic mobilization from tailings in a wetland with neutral drainage. Environ Sci Technol 40(20):6297–6303
DR ATS (2007) Toxicological profile for arsenic. US Department of Health and Human Services, Public Health Service, Atlanta
Elsaied H, Naganuma T (2001) Phylogenetic diversity of ribulose-1, 5-bisphosphate carboxylase/oxygenase large-subunit genes from deep-sea microorganisms. Appl Environ Microbiol 67(4):1751–1765
Fortune WB, Mellon MG (1938) Determination of iron with o-phenanthroline—a spectrophotometric study. Ind Eng Chem 10:0060–0064
Garcia-Dominguez E, Mumford A, Rhine ED, Paschal A, Young LY (2008) Novel autotrophic arsenite-oxidizing bacteria isolated from soil and sediments. FEMS Microbiol Ecol 66(2):401–410
Goldberg S (2002) Competitive adsorption of arsenate and arsenite on oxides and clay minerals. Soil Sci Soc Am J 66(2):413–421
Green HH (1918) Description of a bacterium which oxidizes arsenite to arsenate, and of one which reduces arsenate to arsenite, isolated from a cattle-dipping tank. S Afr J Sci 14:465–467
Harvey CF, Swartz CH, Badruzzaman ABM, Keon-Blute N, Yu W, Ali MA, Jay J, Beckie R, Niedan V, Brabander D, Oates PM, Ashfaque KN, Islam S, Hemond HF, Ahmed MF (2002) Arsenic mobility and groundwater extraction in Bangladesh. Science 298:1602–1606
Hering JG (2005) Contrasting sorption behavior of arsenic(III) and arsenic(V) in suspensions of iron and aluminum oxyhydroxides. Paper presented at the advances in arsenic research, Washington DC, pp 8–24
Hirota R, Kato J, Morita H, Kuroda A, Ikeda T, Takiguchi N, Ohtake H (2002) Isolation and characterization of cbbL and cbbS genes encoding form I ribulose-1, 5-bisphosphate carboxylase/oxygenase large and small subunits in Nitrosomonas sp strain ENI-11. Biosci Biotechnol Biochem 66(3):632–635
Hoeft SE, Lucas F, Hollibaugh JT, Oremland RS (2002) Characterization of microbial arsenate reduction in the anoxic bottom waters of Mono Lake, California. Geomicrobiol J 19(1):23–40
Hoeft SE, Blum JS, Stolz JF, Tabita FR, Witte B, King GM, Santini JM, Oremland RS (2007) Alkalilimnicola ehrlichii sp nov., a novel, arsenite-oxidizing haloalkaliphilic gammaproteobacterium capable of chemoautotrophic or heterotrophic growth with nitrate or oxygen as the electron acceptor. Int J Syst Evol Microbiol 57:504–512
Inskeep WP, Macur RE, Hamamura N, Warelow TP, Ward SA, Santini JM (2007) Detection, diversity and expression of aerobic bacterial arsenite oxidase genes. Environ Microbiol 9(4):934–943
Kojima H, Fukuhara H, Fukui M (2009) Community structure of microorganisms associated with reddish-brown iron-rich snow. Syst Appl Microbiol 32(6):429–437
Krumova K, Nikolovska M, Groudeva V (2008) Characterization of arsenic-transforming bacteria from arsenic contaminated sites in Bulgaria. Biotechnol Biotechnol Eq 22(2):729–735
Lin Z, Puls RW (2000) Adsorption, desorption and oxidation of arsenic affected by clay minerals and aging process. Environ Geol 39(7):753–759
Malasarn D, Saltikov W, Campbell KM, Santini JM, Hering JG, Newman DK (2004) ArrA is a reliable marker for As(V) respiration. Science 306(5695):455
Miziorko HM, Lorimer GH (1983) Ribulose-1, 5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem 52:507–535
Oremland RS, Stolz JF (2003) The ecology of arsenic. Science 300(5621):939–944
Oremland RS, Hoeft SE, Santini JA, Bano N, Hollibaugh RA, Hollibaugh JT (2002) Anaerobic oxidation of arsenite in Mono Lake water and by facultative, arsenite-oxidizing chemoautotroph, strain MLHE-1. Appl Environ Microbiol 68(10):4795–4802
Oremland RS, Stolz JF, Hollibaugh JT (2004) The microbial arsenic cycle in Mono Lake, California. FEMS Microbiol Ecol 48(1):15–27
Oremland RS, Kulp TR, Blum JS, Hoeft SE, Baesman S, Miller LG, Stolz JF (2005) A microbial arsenic cycle in a salt-saturated, extreme environment. Science 308(5726):1305–1308
Paez-Espino D, Tamames J, de Lorenzo V, Canovas D (2009) Microbial responses to environmental arsenic. Biometals 22(1):117–130
Rhine ED, Garcia-Dominguez E, Phelps CD, Young LY (2005) Environmental microbes can speciate and cycle arsenic. Environ Sci Technol 39(24):9569–9573
Rhine ED, Phelps CD, Young LY (2006) Anaerobic arsenite oxidation by novel denitrifying isolates. Environ Microbiol 8(5):899–908
Rhine ED, Ni Chadhain SM, Zylstra GJ, Young LY (2007) The arsenite oxidase genes (aroAB) in novel chemoautotrophic arsenite oxidizers. Biochem Biophys Res Commun 354(3):662–667
Richey C, Chovanec P, Hoeft SE, Oremland RS, Basu P, Stolz JF (2009) Respiratory arsenate reductase as a bidirectional enzyme. Biochem Biophys Res Commun 382(2):298–302
Saltikov CW, Newman DK (2003) Genetic identification of a respiratory arsenate reductase. Proc Natl Acad Sci USA 100(19):10983–10988
Santini JM, vanden Hoven RN (2004) Molybdenum-containing arsenite oxidase of the chemolithoautotrophic arsenite oxidizer NT-26. J Bacteriol 186(6):1614–1619
Santini JM, Sly LI, Schnagl RD, Macy JM (2000) A new chemolithoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Appl Environ Microbiol 66(1):92–97
Santini JM, Sly LI, Wen AM, Comrie D, De Wulf-Durand P, Macy JM (2002) New arsenite-oxidizing bacteria isolated from Australian gold mining environments - Phylogenetic relationships. Geomicrobiol J 19(1):67–76
Scala DJ, Kerkhof LJ (1998) Nitrous oxide reductase (nosZ) gene-specific PCR primers for detection of denitrifiers and three nosZ genes from marine sediments. FEMS Microbiol Lett 162(1):61–68
Senn DB, Hemond HF (2002) Nitrate controls on iron and arsenic in an urban lake. Science 296(5577):2373–2376
Silver S, Phung LT (2005) Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl Environ Microbiol 71(2):599–608
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17(5):517–568
Sun WJ, Sierra-Alvarez R, Fernandez N, Sanz JL, Amils R, Legatzki A, Maier RM, Field JA (2009) Molecular characterization and in situ quantification of anoxic arsenite-oxidizing denitrifying enrichment cultures. FEMS Microbiol Ecol 68(1):72–85
Sun WJ, Sierra-Alvarez R, Milner L, Field JA (2010) Anoxic oxidation of arsenite linked to chlorate reduction. Appl Environ Microbiol 76:6804–6811
Tufano KJ, Reyes C, Saltikov CW, Fendorf S (2008) Reductive processes controlling arsenic retention: revealing the relative importance of iron and arsenic reduction. Environ Sci Technol 42(22):8283–8289
Turner AW (1949) Bacterial oxidation of arsenite. Nature 164(4158):76–77
USEPA (2001) National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring. Fed Regist 66(14):6975–7066
Wang YT, Suttigarn A (2007) Arsenite oxidation by Alcaligenes faecalis strain O1201 in a continuous-flow bioreactor. J Environ Eng 133(5):471–476
Acknowledgments
The work presented here was funded by a U.S. Geological Survey, National Institute for Water Resources 104G grant (2005AZ114G), and by a grant of the National Institute of Environment and Health Sciences-supported Superfund Basic Research Program (NIH ES-04940). The use of trade, product, or firm names in this report is for descriptive purposes only and does not constitute endorsement by the U.S. Geological Survey.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rodríguez-Freire, L., Sun, W., Sierra-Alvarez, R. et al. Flexible bacterial strains that oxidize arsenite in anoxic or aerobic conditions and utilize hydrogen or acetate as alternative electron donors. Biodegradation 23, 133–143 (2012). https://doi.org/10.1007/s10532-011-9493-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-011-9493-x