Abstract
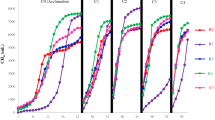
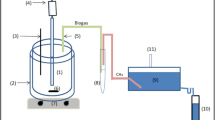
In this paper, we examine variations in the contents of ATP and DNA per unit microbial mass in an acidogenesis of whey permeate. We also introduce a novel approach to estimate microbial mass by measuring ATP and DNA when the ratios of ATP and DNA to microbial mass vary. Acidogenic experiments were performed at 35°C and pH 6.0 in batch mode. The amounts of ATP and DNA per unit microbial mass were not consistent during the incubation except during the post-decay phase. Especially within the exponential phase, each showed a 10-fold difference between maximal and minimal values. In this case, the conventional method which converts ATP or DNA concentration into microbial mass using a fixed conversion factor can give inaccurate results. While the constant ratios of 0.74 mg ATP/g VSS and 1.96 mg DNA/g VSS were determined for the post-decay phase, the ATP and DNA concentrations showed strong linear relationships with the microbial mass (r 2 = 0.99) within the ranges of 0.039–1.078 mg ATP/l and 0.075–2.080 mg DNA/l, respectively. The linear regression equations are as follows: (1) microbial mass concentration (mg/l) = 478.5 × ATP concentration (mg/l) + 293.5, (2) microbial mass concentration (mg/l) = 257.2 × DNA concentration (mg/l) + 250.4. Therefore, changes in the mass of the acidogenic population should be monitored by the combined use of the regression equations obtained in the exponential phase and the constant ratios determined in the post-decay phase. This procedure should be widely applicable to the acidogenesis of dairy processing wastewaters, especially of a highly suspended organic wastewater such as whey.




Similar content being viewed by others
References
Akerlund T, Nordstrom K, Bernander R (1995) Analysis of cell size and DNA content in exponentially growing and stationary-phase batch cultures of Escherichia coli. J Bacteriol 177:6791–6797
APHA-AWWA-WEF (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington DC
Demirel B, Yenigun O (2004) Anaerobic acidogenesis of dairy wastewater: the effects of variations in hydraulic retention time with no pH control. J Chem Technol Biotechnol 79:775–760
Demirel B, Yenigun O, Onay TT (2005) Anaerobic treatment of dairy wastewaters: a review. Process Biochem 40:2583–2595
Demirel B, Yenigun O (2006) Changes in microbial ecology in an anaerobic reactor. Bioresource Technol 97:1201–1208
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric methods for determination of sugars and related substances. Anal Chem 28:350–356
Eiland F, Klamer M, Lind A-M, Leth M, Baath E (2001) Influence of initial C/N ratio on chemical and microbial compostion during long term composting of straw. Microbial Ecol 41:272–280
Foernier D, Schwitzguebel JP, Peringer P (1993) Effect of different heterogeneous inocula in acdidogenic fermentation of whey permeate. Biotechnol Lett 15:627–632
Ghaly AE, Ramkumar DR, Sadaka SS, Rochen JD (2000) Effect of reseeding and pH control on the performance of a two-stage mesophilic anaerobic digester operating on acid cheese whey. Can Agr Eng 42:173–183
Hjort K, Bernander R (1999) Changes in cell size and DNA content in Sulfolobus cultures during dilution and temperature shift experiments. J Bacteriol 181:5669–5675
Hwang S, Hansen CL (1998) Evaluating a correlation between volatile suspended solid and adenosine 5′-triphosphate levels in anaerobic treatment of high organic suspended solids wastewater. Bioresource Technol 63:243–250
Hwang SH, Hansen CL (1992) Performance of upflow anaerobic sludge blanket (UASB) reactor treating whey permeate. T ASAE 35:1665–1671
Ishida A, Yoshikawa T, Nakazawa T, Kamidate T (2002) Enhanced firefly bioluminescence assay of ATP. Anal Biochem 305:236–241
Jorgensen PE, Eriksen T, Jensen BK (1992) Estimation of viable biomass in wastewater and activated sludge by determination of ATP, oxygen utilization rate and FDA hydrolysis. Water Res 26:1495–1501
Kasapagil B, Anderson GK, Ince O (1994) An investigation into the pretreatment of dairy wastewater prior to aerobic biological treatment. Water Sci Technol 29:205–212
Kissalita WS, Lo KV, Pinder KL (1989) Kinetics of whey-lactose acidogenesis. Biotechnol Bioeng 33:623–630
Lee C, Kim J, Hwang S (2006) Optimization of adenosine 5′-triphosphate extraction for the measurement of acidogenic biomass utilizing whey wastewater. Biodegradation 17:347–355
Lee H, Song M, Hwang S (2003) Optimizing bioconversion of deproteinated cheese whey to mycelia of Ganoderma luciderm. Process Biochem 38:1685–1693
Liebeskind M, Dohmann M (1994) Improved method of activated sludge biomass determination. Water Sci Technol 29:7–13
McCarty PL, Smith DP (1986) Anaerobic wastewater treatment. Environ Sci Technol 20:1200–1206
Monoy OH, Vazquez FM, Derramadero JC, Guyot JP (1995) Anaerobic-aerobic treatment of cheese wastewater with national technology in Mexico: the case of ‘El Sauz’. Water Sci Technol 32:59–72
Nelson WH (1991) Physical methods for microorganisms detection. CRC Press, Boca Raton/Ann Arbor/Boston/London
Obata K, Segawa O, Yakabe M, Ishida Y, Kuroita T, Ikeda K, Kawakami B, Kawamura Y, Yohda M, Matsunaga T, Tajima H (2001) Development of a novel method for operating magnetic particles, magtration technology, and its use for automating nucleic acid purification. J Biosci Bioeng 91:500–503
Rajeshwari KV, Balakrishnan M, Kansal A, Lata K, Kishore VVN (2000) State-of-the-art of anaerobic digestion technology for industrial wastewater treatment. Renew Sust Energ Rev 4:135–156
Robertson BR, Button DK, Koch AL (1998) Determination of the biomasses of small bacteria at low concentrations in a mixture of species with forward light scatter measurements by flow cytometry. Appl Environ Microbiol 64:3900–3909
Shuler ML, Kargi F (2002) Bioprocess engineering: basic concepts, 2nd edn. Prentice Hall PTR, Upper Saddle River
Shuler ML, Tsuchiya HM (1975) Cell size as an indicator of changes in intracellular composition of Azotobacter vinelandii. Can J Microbiol 21:927–935
Speece RE (1996) Anaerobic biotechnology for industrial wastewaters. Archae Press, Nashville, TN
Stoeck T, Duineveld GCA, Kok A, Albers BP (2000) Nucleic acids and ATP to assess microbial biomass and activity in a marine biosedimentary system. Mar Biol 137:1111–1123
van den Berg L, Kennedy KJ (1992) Dairy waste treatment with anaerobic stationary fixed film reactors. In: Malina JF, Pohland FG (eds) Design of anaerobic processes for the treatment of industrial and municipal wastes. Technomic Publishing Company, Pennsylvenia
Yang K, Yu Y, Hwang S (2003) Selective optimization in thermophilic acidogenesis of cheese-whey wastewater to acetic and butyric acids: partial acidification and methanation. Water Res 37:2467–2477
Yang S, Tang I (1991) Methanogenesis from lactate by a co-culture of Clostridium formicoaceticum and Methanosarcina mazei. Appl Microbiol Biotechnol 35:119–123
Yu Y, Hansen CL, Hwang S (2002) Biokinetics in acidogenesis of highly suspended organic wastewater by adenosine 5′ triphosphate analysis. Biotechnol Bioeng 78:147–156
Zapsalis C, Beck RA (1986) Food chemistry and nutritional biochemistry. Macmillan Publishing Co, New York
Acknowledgements
This work was financially supported in part by the Korea Science and Engineering Foundation through the Advanced Environmental Biotechnology Research Center (R11–2003–006) at Pohang University of Science and Technology and by the BK-21 program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J., Lee, C., Shin, S.G. et al. Correlation of microbial mass with ATP and DNA concentrations in acidogenesis of whey permeate. Biodegradation 19, 187–195 (2008). https://doi.org/10.1007/s10532-007-9125-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-007-9125-7