Abstract
With the rapid pace of global warming, there is an urgent need to understand survival responses to climate, particularly for large mammals that are already experiencing population declines associated with anthropogenic pressures such as poaching and habitat loss. We tested hypotheses about the interactive effects of local climatic anomalies (variations around a long-term mean) and proximity to edge of protected area boundaries on seasonal adult and juvenile survival in a population of 2,385 individually identified giraffes monitored over 8 years in the Tarangire Ecosystem of northern Tanzania. Temperature anomalies were positively correlated with seasonal survival of adult giraffes, suggesting these megaherbivores are adapted to hot conditions. Higher seasonal rainfall anomalies were negatively correlated with both juvenile and adult survival, and greater vegetation greenness was associated with lower adult survival. During seasons of anomalously high rainfall and vegetation greenness, higher parasite and disease abundance, poorer-quality nutrition in forage, and higher predation risk may all play a role in lowering giraffe survival. Furthermore, climate-associated reduction in survival was most pronounced during the short rainy season for adult giraffes living closer to the edge of protected areas, indicating that the influence of climate anomalies may be exacerbated by anthropogenic edge effects such as poaching or livestock keeping. Precipitation in East Africa is projected to increase substantially, with a greater proportion of rain falling during heavy events in the short rainy season, which may threaten persistence of giraffes in one of Earth’s most important landscapes for large mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Demographic responses to climate are complex and vary among sexes and ages within a species (Clutton-Brock et al. 1985; Gaillard et al. 1997; Modafferi and Becker 1997) arising from differences in life-histories, physiology, developmental stage, and geographical area (Isaac 2009; Saether et al. 2013; Paniw et al. 2018, 2021). Vital rates also are affected by anthropogenic pressures like hunting (Milner et al. 2007; Wittemyer et al. 2013). An expanding body of literature is elucidating ways in which variation in temperature, rainfall, and primary productivity are linked to heat or cold stress, disease, changes in food quality, and human-wildlife conflicts, thereby interactively influencing demography (e.g., Sauer and Boyce 1983; Owen-Smith 1990; Watts and Holekamp 2009; Mumby et al. 2015; Woodroffe et al. 2017; Cohen et al. 2020, Rabaiotti et al. 2021; Titcomb et al. 2021; Paniw et al. 2022). Such information helps scientists and wildlife managers understand the processes driving differential population responses to climate change.
Large mammals are ecosystem engineers and keystone species through their ‘outsized’ effects on ecosystems (Fritz et al. 2002; Doughty et al. 2016). They are particularly vulnerable to extirpation and extinction due to their vast land requirements and potential for conflicts with humans (Alroy 2001; Galetti et al. 2018). In the face of increasing human presence, the additional stressor of extreme fluctuations in climate could push already declining populations of large mammals closer to extinction, but little is known about the interactive effects of climate and anthropogenic pressures on demography of these animals. Very large-bodied animals such as megaherbivores, defined as plant-eating animals that can exceed 1,000 kg in mass (Owen-Smith 1992), may be especially susceptible to climate anomalies (variations from a long-term mean) as witnessed in elephants (e.g., African savanna elephants Loxodonta africana: Foley et al. 2008, Asian elephants Elaphus maximum; Mumby et al. 2013). Giraffes (Giraffa camelopardalis) are endemic African megaherbivores, and research has demonstrated how their demography is influenced by human activities (Strauss et al. 2015; Lee 2018) but their survival responses to climate variation have not been studied. Giraffes are long-lived (approximately 30 year; Lee et al. 2020) and slow breeding (Lee and Strauss 2016). Adult female survival makes the greatest contribution to population growth rates (Lee et al. 2016a; Strauss et al. 2015), but juvenile survival can also be important to population dynamics (Suraud et al. 2012; Lee et al. 2016a). Investigating survival responses to seasonal and spatial interactions with climate variation allows us to pinpoint fine-scale temporal climatic processes influencing population dynamics of this megaherbivore.
Giraffes respond to many of the factors that drive population dynamics in other tropical and sub-tropical species, such as changes in vegetation, predation risk, disease prevalence, and poaching pressure (Turner et al. 2013; Strauss et al. 2015; Lee et al. 2016b; Muller 2018), yet giraffes are singular in several respects. Very large animals can overheat (e.g. Asian elephants; Mumby et al. 2013), but the giraffe’s body shape and anatomy facilitate evaporative heat loss (Langman et al. 1979; Mitchell and Skinner 2004; Mitchell 2021). Like other herbivores, seasonality mediates resource use and movements of giraffes because their primary forage fluctuates in palatability and nutrient quality across space and time (Pellew 1984; Fennessey 2004; Levi et al. 2022), but giraffes can roam across vast areas (Knüsel et al. 2019). Like many savanna animals, giraffes are susceptible to mortality from diseases such as anthrax (Hampson et al. 2011; Turner et al. 2013) and Rift Valley fever virus (Evans et al. 2008; Fischer et al. 2013), with outbreaks influenced by heavy rainfall (Wensman et al. 2015; Mwakapeje et al. 2018). Fecal-oral parasites are transmitted among animals aggregated at water sources (Mwakapeje et al. 2018; Titcomb et al. 2021), but giraffes are less water dependent than other ruminants (Mitchell 2021) and thus are less apt to congregate at water points (Titcomb et al. 2021). Therefore, survival responses of giraffes to fluctuations in temperatures, rainfall, or primary productivity may diverge from those seen in smaller ungulates or other megaherbivores.
Our aim here was to test hypotheses about how local seasonal climatic anomalies influence giraffe survival along a gradient of human presence in an East African savanna landscape. The literature suggests several potential mechanisms whereby local climatic anomalies might affect survival rates of giraffes. Temperatures may exert direct effects on animals via thermoregulation and heat or cold stress (Fuller et al. 2010; Speakman and Król 2010). Disease transmission is often linked to rainfall or temperature extremes (Altizer et al. 2006; Cohen et al. 2020) and fluctuations in rainfall levels influence foliage abundance and/or nutrient availability in leaves (Breman and de Wit 1983; Olff et al. 2002). Each of these might affect giraffe health, either independently or synergistically. Other known sources of giraffe mortality include natural predation on juveniles (Strauss and Packer 2013; Lee et al. 2016a, b) but rarely on adults (B. Kissui, unpubl. data) and poaching of all age classes (Kiffner et al. 2015; Strauss et al. 2015) which might vary seasonally and spatially due to differential access or availability.
We analyzed a continuous 8-year dataset of 2,385 individually identified Masai giraffes (subspecies G. c. tippelskirchi or species G. tippelskirchi, Matschie 1898) inhabiting the Tarangire Ecosystem of northern Tanzania (Fig. 1) to investigate how seasonal anomalies in temperature, rainfall, and vegetation greenness (an index of primary productivity) were correlated with seasonal survival by sex and age class, and in interaction with proximity to human-dominated areas. We explored various moving windows of climatic variation (current season, previous season, and current plus previous seasons) to account for potential lagged or cumulative effects on demography, and tested cohort-like effects of climate during conception and early gestation (12 to 16 months prior to birth) on subsequent juvenile survival rates. We predicted the following directional effects of climatic anomalies:
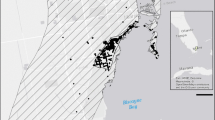
Masai giraffe demographic study area in the Tarangire Ecosystem of northern Tanzania. Purple points are giraffe locations from mark-resight surveys conducted from 2012‒2020. White lines are survey tracks. Gray polygons are protected areas Tarangire National Park, Manyara Ranch, and Burunge and Randilen Wildlife Management Areas. Dark gray stars are livestock herder bomas (temporary settlements), black polygons are towns comprising concrete structures, and black lines are tarmac roads. Open circle on inset of Africa shows approximate location of the Tarangire Ecosystem
H1
Negative temperature effects on survival due to overheating.
H2
Negative rainfall effects on survival due to increased parasite and disease prevalence (affecting all age classes) and/or increased predator stalking cover (affecting juveniles).
H3
Positive vegetation greenness effects on survival from increases in forage.
H4
Negative rainfall effects during wet seasons for giraffes roaming closer to the edge of protected areas due to increased disease from livestock and/or poaching.
Materials and methods
Study area
Our study area included the northern portion of Tanzania’s Tarangire National Park, two adjacent Wildlife Management Areas, and Manyara Ranch, which together form the core of giraffe habitat in the Tarangire Ecosystem (Fig. 1). All parts of our study area were unfenced and connected by movements of giraffes (Lee and Bolger 2017). Latitude is 2°S to 5°S, longitude is 35°E to 37°E, and elevation ranges from 950 to 1200 m. Vegetation was predominantly semi-arid savanna composed of open grassland, woodland, riverine forest, and dense bushland (Pratt et al. 1966).
Field data collection
Pelage patterns can be used for identification of giraffes because they are individually unique and unchanging so their ‘marks’ cannot be lost (Foster 1966). We employed active photographic encounter surveys, systematically collecting data according to a strict sampling protocol to produce equal sampling effort across time and space. We collected giraffe photographic data from January 2012 to February 2020 using the robust design, with two replicated (secondary) surveys during each primary sampling occasion, to improve precision of demographic parameter estimates (Pollock 1982). In northern Tanzania, there are three precipitation seasons per year [short rains = Oct–Jan, long rains = Feb–May, and dry season = Jun–Sep], so we assigned our sampling occasions to occur at the end of each of the three seasons to capture seasonal effects on survival. We conducted a total of six independent surveys per year (three primary sampling occasions × two secondary sampling events within each primary occasion).
During surveys, we drove the same network of fixed route road transects throughout our study area (Fig. 1). We maintained a driving speed between 15 and 20 km/h on all transects, and all surveys were conducted by the same two observers (MLB and DEL). We sampled each road segment only one time in a given event, and systematically shifted the order and direction in which we sampled sites and road transects to reduce sampling biases. Our sampling design of 14 days per primary sampling occasion (driving all roads twice), with 3.5 months between sampling occasions, proved to be effective in providing precise estimates of demographic rates (Lee et al. 2016a, b; Lee and Bolger 2017). The study area size of ~ 1,010 km2 was large enough to encompass multiple home ranges of giraffes (mean 114 and 150 km2 for adult females and adult males, respectively; Knüsel et al. 2019), yet small enough to survey quickly to meet assumptions of closed populations for robust design. Road density throughout our study area was high (0.42 km/km2) relative to giraffe home range size, and our capture and recapture probabilities were sufficient (> 0.2). After the first six surveys, we had identified 96% of the adult giraffes in our study area (Bond et al. 2021), and each subsequent survey was subsampling from the total known population, as well as identifying newly born individuals.
When we encountered any giraffes, we ‘marked’ newly observed individuals or resighted previously observed animals by slowly approaching and photographing the giraffe’s right side. We recorded sex (male, female), GPS location, and age class (newborn 0–3 months, subadult 4 months to 3 years, or adult > 3 years) based on a suite of physical characteristics (Strauss et al. 2015). We matched giraffe identification images using WildID, a freely downloadable computer program that matches large datasets of giraffe images collected using our protocols with low error rates (Bolger et al. 2012).
Local climate covariates
Our climate covariates included seasonal land surface temperature, rainfall, and normalized difference vegetation index (NDVI), a measure of vegetation greenness reflecting primary productivity. We expected that seasonal giraffe survival rates would respond to deviations in the local climate rather than to the raw values, so we used standardized seasonal anomalies as covariates. The covariates thus expressed local climate seasonal anomalies specific to each of the three annual seasons with a mean equal to zero and a standard deviation of one, making it easier to discern ‘normal’ versus ‘unusual’ conditions. Details on data sources and raw values and anomalies for climate covariates are provided in Supporting Information and Figures S1-S2.
In our demographic modeling we included various moving time windows of the covariates to account for cumulative and lag effects on giraffe survival rates: the climate anomalies of the current season; one prior season; the current and one prior season combined; and—for juvenile survival—cohort-like effects of local climatic conditions during conception and early pregnancy, from 12 to 16 months prior (giraffe gestation period is ~ 15 months; del Castillo et al. 2005). In preliminary analysis we found covariates for 1 year prior (three seasons combined) poorly fit the data.
Estimating survival
We summarized the giraffe data into individual encounter histories, analyzed the encounter histories, and tested hypotheses following general methods of mark-resight data analysis (Burnham and Anderson 2002; Amstrup et al. 2006; Cooch and White 2019) using the package RMark (Laake 2013) for R (R Core Development Team 2017). We created two encounter history datasets: (1) adults only: a known-sex, unknown-age group composed of giraffes first detected as adults (> 3 years), and (2) juveniles only: a known-age, known- and unknown-sex group composed of giraffes first detected as newborns (0–3 months). For both datasets, we estimated apparent survival probabilities, the probability that an individual alive in season t was also alive in season t + 1, given the individual remained on the study area. We grouped individuals by sex (for juveniles this included groups for males, females, and unknown sex) to test for sex differences in responses to climate conditions. For juveniles, we additionally included effects of age and age2 as well as season of birth in all models as these were included in top-ranked juvenile survival models in previous analyses (Lee et al. 2017).
We utilized the Pradel robust design model (with two secondary sampling events per seasonal sampling occasion) to provide estimates of seasonal apparent survival (S), temporary emigration (γ” and γ’), and detection probabilities (p and c) (Pradel 1996). We assumed a random emigration model, where a giraffe is as likely to leave as to enter the population (γ” equal to γ’, hereafter γ), and equal detection rates for the first and subsequent detection in a primary occasion (p equal to c, hereafter p). We also included spatial factors of mean distance to the edge of the study area as a covariate to detection, and mean distance to the edge of protected area as a covariate to survival. We tested the fit of our data to a fully time-dependent model by calculating median ĉ (Cooch and White 2019). We ranked models using Akaike’s Information Criterion corrected for small samples (AICc) and used model weights (W) as a metric for strength of evidence supporting a given model as the best description of the data (Burnham and Anderson 2002).
Some individual giraffes reside closer to the edge of our study area boundaries, which may influence their detection probabilities due to temporary emigration from the study area. For each survey, we calculated every giraffe’s distance from the edge of the study area (in km), defined as 1 km from the outer edge of each of the outermost roads that we surveyed, using the Generate Near Table tool in ArcMap 10.8.1 (ESRI 2020). We included the mean distance (MeanDistSA), and a season × MeanDistSA interactive effect, as an individual spatial covariate in γ and p following spatial capture-recapture methods (Royle et al. 2014). Additionally, giraffes residing closer to the edges of protected areas may be subjected to increased levels of poaching (Lee et al. 2016a), and increased exposure to livestock carrying parasites and diseases (VanderWaal et al. 2014; Aruho et al. 2021), whereas juveniles living closer to the edges may evade predation because of lower predator densities outside the protected areas (Lichtenfeld 2005). Therefore, we included the mean of each animal’s survey-specific distance from the edge of the protected area boundaries (MeanDistPA) as an individual spatial covariate to survival, and a season × MeanDistPA interactive effect.
We adopted a multistep approach to build and select the best model structures for our analyses, as follows: (1) fixing S at full parameterization and selecting the best model of sex, year, and season, as well as age and age2 effects for juveniles, on temporary emigration (γ) and detection (p) probabilities; then (2) fixing temporary emigration and detection based on results from step 1 and selecting the best model with sex, year, and season, as well as age and age2 effects for juveniles, on S; then (3) fixing S, temporary emigration, and detection based on results from step 2 and testing for spatial effects on temporary emigration and detection; and finally (4) fixing temporary emigration and detection based on results from step 3, adding effects of season of birth and adding and testing our climate covariates of temperature, rainfall, NDVI, their interactions with sex and season (and age, age2, and season of birth effects for juveniles), and spatial effects on S. For juveniles, we also added a step 5: fixing temporary emigration and detection based on results from step 4 and testing for age and climate covariate effects during conception (12–16 months prior to survey). This multistep approach allowed us to gradually increase the complexity of our model components, which led to the most parsimonious model structure to test our main hypotheses.
Results
We recorded substantial variation in seasonal anomalies of temperature, rainfall, and NDVI during the period beginning approximately one decade prior to and through to the end of our study (Supporting Information Fig. S2). No pair of local climate covariates were significantly correlated (Pearson’s product-moment correlation = rainfall and temperature: -0.36, 95% CI = -0.67 to 0.05, t = -1.81, df = 22, P = 0.08; rainfall and NDVI: 0.05, 95% CI = -0.365 to 0.44, t = 0.23, df = 22, P = 0.82; temperature and NDVI: -0.33, 95% CI = -0.64 to 0.089, t = -1.62, df = 22, P = 0.12, Supporting Information Fig. S3), therefore our models included various combinations of the covariates.
We used encounter histories for 1,248 adult giraffes (757 females, 491 males) and 1,137 juveniles first observed as newborns (387 females, 427 males, 323 unknown sex) to estimate the sex-specific influence of local climate anomalies on seasonal survival rates. We found no evidence for lack-of-fit in the encounter history data, as estimated median ĉ was close to 1 for both datasets (adults = 1.19 with sampling SE = 412; juveniles = 1.02 with sampling SE = 391). Therefore, we retained ĉ = 1.0 for all model selection (Burnham and Anderson 2002).
Temporary emigration and detection models that included mean distance to the edge of the study area ranked substantially higher (Supporting Information Table S1-2, Figs. S4-S5), so all climate covariate modeling included this spatial effect. Males showed higher temporary emigration rates than females (Supporting Information Figs. S4-S5), and unknown-sex juveniles showed higher temporary emigration and lower survival rates than known-sex juveniles (Supporting Information Fig. S5). Neonatal giraffes are difficult to determine sex, so it is likely that many unknown-sexed calves experienced mortality before we were able to assign sex.
Adult survival
The three top-ranked climate covariate models of apparent survival for the adult dataset all included a negative effect of higher NDVI anomalies in the season of survey (H3): the most parsimonious model carried more than four times the weight of the first model in the set without this covariate (Table 1). Weighted models (W > 0.01) included a negative effect of distance from the edge of the protected areas (MeanDistPA) and an interaction between season and MeanDistPA. The most pronounced adverse effect of higher NDVI anomalies was during the short rains for adults living closer to the edge of the protected areas (Table 1; Fig. 2). Weighted models also included a negative influence on adult survival of higher rainfall anomalies in the current survey (H2) with similar directional effects and seasonal interactions with MeanDistPA as NDVI (H4), as well as positive seasonally variable effects of higher temperature anomalies in the current season (H1) (Table 1; Fig. 2). No cumulative and lag effects of climate anomalies were evident. Adult males always had lower survival than females (Fig. 2).
Juvenile survival
The most parsimonious juvenile survival model included positive effects of age, and a negative influence of higher rainfall anomalies in the season of survey (H2) which varied by season and by MeanDistPA (H4). This model indicated positive effects of more rainfall during the short rains for juveniles living farther from the edge of the protected areas (Table 1) but the effect size was slight (Fig. 3). All weighted climate covariate models for juvenile survival included a negative effect of rainfall anomaly during the current season (Table 1). Climate conditions during gestation did not significantly influence juvenile survival, and we found little support for models that included temperature (H1) or NDVI anomalies (H3), or cumulative and lag effects.
Discussion
We documented biologically significant effects of local climate anomalies on survival rates of Masai giraffes in the Tarangire Ecosystem—climatic factors that are projected to fluctuate more frequently and intensely in the future in East Africa (McSweeney et al. 2010; Cook et al. 2020). Our study demonstrated that adult—but not juvenile—survival was positively correlated with higher temperatures. We also showed significant negative effects of anomalously high rainfall on both adult and juvenile survival rates, except for juvenile survival during the short rains. Contrary to our expectations, greener vegetation was correlated with lower adult survival, but had no detectable influence on juveniles. Our results also revealed seasonal spatial effects on giraffe survival related to proximity to the edge of protected area boundaries during the short rains. Animals roaming closer to the edge (where poaching and livestock-mediated disease risk is greater) had lower survival than animals roaming farther from human-dominated areas, and this was especially pronounced for adults (but not juveniles) when rainfall was anomalously high during the short rainy season. These complex and varied interactive effects of seasonal climatic variation and spatial factors on giraffe vital rates demonstrate how investigating correlations between climate and demography across both space and time can help illuminate some of the processes underlying population persistence in an era of rapid climate change.
Temperature
High ambient temperatures constrain dissipation of body heat (Speakman and Król 2010), which may be especially apparent in hot, arid environments (Fuller et al. 2010; Shrestha et al. 2014). We had predicted that being megaherbivores, both juvenile and adult giraffes would suffer from overheating and increased mortality during higher temperature anomalies (H1) but found that warmer seasons were correlated with higher adult survival.
Mitchell and Skinner (2004), Mitchell et al. (2017), and Mitchell (2021) provided comprehensive overviews of thermoregulation in giraffes and suggested that giraffes are more adapted to tolerate hotter rather than colder temperatures through an array of physiological and behavioral mechanisms (Fuller et al. 2010). At higher ambient temperatures, giraffes in Namibia sought shade or stood longitudinally to the sun which decreases radiant heat gain (Kuntsch and Nel 1990) and their locomotor activity was lower during hotter daytime periods (Hart et al. 2020). Mitchell et al. (2017) proposed that the giraffes’ unique narrow physique with long necks and legs enables convective and evaporative heat loss, and the structure of their nasal cavities and the carotid rete (Mitchell and Lust 2008) contribute to cooling. Giraffes also dissipate heat at their pelage spot patches, and have sweat glands (Mitchell et al. 2017; Mitchell 2021). In contrast, elephants lack sweat glands (Hiley 1975) and Asian elephants showed significantly lower survival during the hottest months in a 35-year demography study in Myanmar (Mumby et al. 2013). Overall, Mitchell et al. (2017) posited that giraffes are more likely to have difficulty retaining rather than dissipating heat and our results generally support this supposition, at least for adults. Heat tolerance, or conversely cold intolerance, of giraffes may increase with developmental growth, as we found no effects of temperature on juvenile survival. The higher temperature anomalies experienced by giraffes during our study period appeared to be within their physiologically tolerable envelope. However, temperatures are expected to continue rising in the Tarangire Ecosystem and elsewhere in the species’ range (IPCC 2022), and should extreme heat waves occur in the future, research may reveal threshold temperatures above which adverse effects on giraffes become evident.
Rainfall and NDVI
Bartzke et al. (2018) asserted that rainfall is the principal driver of the population dynamics of savanna herbivores by mediating plant biomass production and nutrient concentration, as well as diseases. In our analysis, we separated the influences of rainfall and NDVI anomalies, which were not correlated in our study area. We had predicted that anomalously high rainfall might increase giraffe mortality, by contributing to higher predator stalking cover and/or higher rates of infectious disease (H2), and our results supported our prediction for both juveniles and adults.
Predator stalking cover
—Our juvenile survival results agree with a study of a Masai giraffe population in the Masai Mara of Kenya where road-based counts showed the number of newborns seen per adult female was highest in birth years preceded by low wet-season rainfall, and lowest in years preceded by high wet-season rainfall (Ogutu et al. 2013). Some rainfall during dry seasons was correlated with higher giraffe calf counts in Kruger National Park, South Africa (Owen-Smith et al. 2005), as well as higher calf densities in the Masai Mara (Ogutu et al. 2008). However, densities of calves in the Masai Mara declined during excessive dry-season rainfall (Ogutu et al. 2008), indicating similar negative effects on calves of anomalously high rainfall as we found in our Tarangire population. Adult giraffes in the Tarangire Ecosystem are rarely killed by predators (B. Kissui, unpubl. data) but calves are vulnerable, thus higher than normal rainfall could impact juvenile giraffe survival either by increasing vegetative cover for stalking predators (Hopcraft et al. 2005) or by facilitating infectious disease (see below). We detected the strongest adverse effects of high rainfall on juvenile survival during the long rains, suggesting that stalking cover height as mediated by increased rainfall may indeed play a role in predation risk.
Infectious diseases
—Higher rainfall conditions may have increased parasite transition in both juvenile and adult giraffes, impairing health or causing mortality of already less-healthy individuals (Titcomb et al. 2021). More rainfall was associated with increased rates of infectious diseases in other African ungulates: months of higher rainfall were correlated with greater lungworm intensity and Coccidia infection in Grant’s gazelles (Nanger granti) in East Africa (Williams et al. 2017). Internal parasites found in giraffes include flatworms, nematodes, and bacteria, some of which are transmitted through tick bites (Shorrocks 2016). Tick abundance tends to increase with above-average rainfall (Keesing et al. 2018). Mosquito-borne diseases that infect giraffes, like Rift Valley fever virus (Fischer et al. 2013), can be triggered by extreme or prolonged rains (Evans et al. 2008) during which large numbers of floodwater mosquitos emerge (Wensman et al. 2015; de Glanville et al. 2022). In Kenya, giraffes did not carry detectable Rift Valley fever virus antibodies except during a severe outbreak caused by heavy flooding (Evans et al. 2008). Rift Valley fever virus induces abortion in pregnant animals and high mortality rates of neonates (Fischer et al. 2013). Giraffes are also susceptible to mortality from anthrax, caused by Bacillus anthracis (Hampson et al. 2011; Turner et al. 2013), and outbreaks often occur during wetter-than-average conditions for a system, when flooding unearths spores (Hampson et al. 2011; Huang et al. 2022). Huang et al. (2022) noted that the positive relationship between rainfall and anthrax cases occurs after short time intervals on the order of 1–2 weeks, consistent with water aggregation and transport of spores that would directly and immediately affect giraffe survival. Both Rift Valley fever virus (Wensman et al. 2015; de Glanville et al. 2022) and B. anthracis (Mwakapeje et al. 2018) were detected in humans, livestock, and wildlife in Arusha and Manyara regions of northern Tanzania during our study period, and anthrax was found in giraffe in the nearby Serengeti Ecosystem (Mwakapeje et al. 2018; Hampson et al. 2011), although no targeted research has been conducted on disease-caused mortality of giraffes in the Tarangire Ecosystem, with the exception of a study of skin disease on giraffe forelegs (Bond et al. 2016). However, gastro-intestinal parasites in giraffe dung in the Tarangire Ecosystem were elevated during the rainy seasons compared with the dry (Mkessa 2022). Titcomb et al. (2021) showed that eggs of ungulate fecal-oral parasites in central Kenya were more abundant in wetter locations during higher rainfall, and these conditions may have contributed to elevated giraffe mortality we documented in our study.
In contrast to adult giraffes, juvenile survival was not adversely affected by higher rainfall during the short rains. However, similar to adults, survival of young giraffes during the short rains was lower closer to the edges of the protected areas. This result suggests that some factor other than rainfall is associated with reduced juvenile survival at the edges of protected areas in the short rains (see seasonal spatial effects below). It may be that livestock-mediated parasites emerge in the short rains that follow the dry season (Mkessa 2022), which negatively affects all age classes even during average local climate conditions, but that this negative effect is exacerbated only in adults during heavier rains.
Plant nutrient concentration
—Anomalously high vegetation greenness was significantly correlated with lower adult giraffe survival, which differed from our expectations (H3). Adverse effects of vegetation greenness might be explained by excessive plant growth that dilutes plant nutrient concentration (Olff et al. 2002). Experimental evidence showed that providing African savanna tree seedlings with supplemental water reduced the mean nitrogen, phosphorus, and potassium concentrations in leaves (Barbosa et al. 2014). Thus, plant growth that increases foliage biomass (reflected in higher values of NDVI) actually decreases leaf nutrient concentration, which may reduce forage quality for selective browsers such as giraffes (Shorrocks 2016), potentially impacting survival of individuals in suboptimal health conditions.
The lack of this effect detected in juvenile giraffes could be because nursing calves are buffered against variations in food resources (Fryxell 1987). In Southern Sudan, mortality of white-eared kob (Kobus kob leucotis) calves did not differ between a year of substantial precipitation and a year of drought (Fryxell 1987). In our case, browse quality may have been reduced during seasons of anomalously high NDVI but mother giraffes may still have been able to transfer needed nutrients to their offspring via their milk—this may have enabled their calves to survive at the expense of their own health.
Seasonal spatial effects
Spatial effects can synergistically interact with climate to influence vital rates. One of our objectives was to determine whether seasonal survival of giraffe adults and juveniles was affected by distance to the edge of the protected areas where poaching pressure and livestock-mediated disease risk is higher (H4). We had expected that survival as a function of distance to the edge of protected areas would be lower during rainy seasons because mud can hamper vehicular anti-poaching patrols and human-dominated areas have high densities of livestock that are disease and parasite reservoirs (VanderWaal et al. 2014). We indeed found lower survival close to protected area edges during the short rains (Figs. 2 and 3). Natural predator densities, which primarily affect juveniles, are lower outside the protected areas (Lichtenfeld 2005). However, the interactive effects of protected area and season indicated lower survival for juveniles closer to people during the short rains and a slightly positive effect of more rain on survival of all juveniles in the short rains, regardless of location.
Increased poaching of giraffes—especially of adults—during the short rains might coincide with the beginning of the rainy season when ranger patrols are hampered by wet conditions, or giraffe survival might be impacted when livestock-transmitted parasites emerge after the dry season. Indeed, giraffe gastrointestinal parasite intensity was significantly higher during the rainy seasons than the dry season in the Tarangire Ecosystem, with the highest parasite egg counts in giraffe dung samples recorded during the short rains (Mkessa 2022). Targeted research on seasonal patterns of bushmeat poaching and parasite emergence, and whether the parasites that emerge during the short rains are associated with livestock as well as lethal to giraffes, might elucidate specific mechanisms affecting seasonal survival during local climate anomalies. Nevertheless, accounting for seasonal spatial effects revealed potential climate-correlated anthropogenic sources of mortality for adults and juveniles that warrants further investigation.
Climatic variation exacerbates existing anthropogenic threats in other regions of Africa (Watts and Holekamp 2009; Rabaiotti et al. 2021). Rabaiotti et al. (2021) showed that high ambient temperatures were associated with increased risks of African wild dogs (Lycaon pictus) being killed by people. A negative effect of rainfall on recruitment of spotted hyenas (Crocuta crocuta; Watts and Holekamp 2009) was attributed to retaliatory killing by pastoralists in response to livestock depredation during wetter periods when natural prey became more dispersed. In the Tarangire Ecosystem, increasingly wet and muddy conditions may facilitate poachers along the boundaries of protected areas, or boundaries represent livestock-mediated disease and parasite reservoirs, contributing to further losses in this giraffe population.
Across East and southern Africa, the range of most subspecies of giraffes, mean annual temperatures have generally increased over the past several decades, and cold extremes have decreased in Southern Africa; these projections are expected to continue (IPCC 2022). Despite increasingly frequent droughts, mean annual rainfall also has increased across many areas of southern and East Africa, and this trend also is projected to continue. Demography studies of the southern giraffe populations should test the effects of local climate anomalies on survival rates to determine whether the patterns we detected in Tarangire are similar across the latitudinal range of the species.
Conclusions
Species-specific responses to climate variation are expected to vary by life-history (Isaac 2009; Paniw et al. 2021), as large, long-lived, slow-reproducing mammals with delayed maturity are theoretically more buffered from environmental variation than short-lived, fast-reproducing species (Saether et al. 2013; Paniw et al. 2018). Survival of adult giraffes in the Tarangire Ecosystem was positively affected by higher seasonal temperature anomalies, possibly because their physical architecture is well-suited to heat dissipation, but adversely affected by higher rainfall anomalies and vegetation greenness, demonstrating that climate change could indeed act as a mechanism for population declines of this megaherbivore. Like other long-lived, slow-reproducing mammals, adult female giraffes are especially important to population growth and sustainability (Lee et al. 2016a). Climate anomalies that cause higher mortality of this critical segment of the population could reduce giraffe numbers if such anomalies occur more frequently. Extreme weather events in East Africa are becoming more common: annual and wet season severe droughts and floods in the Masai Mara have doubled since 1990 compared to the previous 30 years (Bartzke et al. 2018). Both rainfall and temperatures are projected to rise in Tanzania over the coming decades (Cook et al. 2020), with a greater proportion of rain falling in heavy events during the short rainy seasons (McSweeney et al. 2010; Cook et al. 2020) which is likely to threaten the sustainability of giraffe populations in this region. Some causes of adult giraffe mortality, such as poaching for meat and body parts, can be more readily ameliorated than climatic conditions, by expanding anti-poaching measures and developing alternative livelihoods for people (Lee 2018). Such management actions will become increasingly important for conservation of giraffes and other imperiled species as the many stressors of climate change intensify in the coming years.
References
Alroy J (2001) A multispecies overkill simulation of the end-pleistocene megafaunal mass extinction. Science 292:1893–1896
Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P (2006) Seasonality and the dynamics of infectious diseases. Ecol Lett 9:467–484
Amstrup SC, McDonald TL, Manly BFJ (eds) (2006) Handbook of capture–recapture analysis. Princeton University Press, Princeton
Aruho R, MacLeod ET, Manirakiza L, Rwego IB (2021) A serological survey of brucellosis in wildlife in four major national parks of Uganda. BMC Veterinary Res 17:95–105
Barbosa ERM, Tomlinson KW, Carvalheiro LG, Kirkman K, de Bie S, Prins HTH, van Gangevelde F (2014) Short-term effect of nutrient availability and rainfall distribution on biomass production and leaf nutrient content of savanna tree species. PLoS ONE 9:e92619
Bartzke GS, Ogutu JO, Mukhopadhyay S, Mtui D, Dublin HT, Piepho H-P (2018) Rainfall trends and variation in the Maasai Mara ecosystem and their implications for animal population and biodiversity dynamics. PLoS ONE 13:e0202814
Bolger DT, Morrison TA, Vance B, Lee DE, Farid H (2012) A computer-assisted system for photographic mark–recapture analysis. Methods Ecol Evol 3:813–822
Bond ML, Strauss MKL, Lee DE (2016) Soil correlates and mortality from Giraffe skin disease in Tanzania. J Wildl Dis 52:953–958
Bond ML, König B, Ozgul A, Farine DR, Lee DE (2021) Socially defined subpopulations reveal demographic variation in a giraffe metapopulation. J Wildl Manage 85:920–931
Breman H, de Wit CT (1983) Rangeland productivity and exploitation in the Sahel. Science 221:1341–1347
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretical approach. Springer-Verlag, New York, New York, USA
Clutton-Brock T, Major M, Guinness FE (1985) Population regulation in male and female red deer. J Anim Ecol 54:831–846
Cohen JM, Sauer EL, Santiago O, Spencer S, Rohr JR (2020) Divergent impacts of warming weather on wildlife disease risk across climates. Science 370:933
Cooch EG, White GC (2019) Program MARK: a gentle introduction. 19th edition
Cook KH, Fitzpatrick RGJ, Liu W, Vizy EK (2020) Seasonal asymmetry of equatorial east african rainfall projections: understanding differences between the response of the long rains and the short rains to increased greenhouse gases. Clim Dyn 55:1759–1777
de Glanville WA, Nyarobi JM, Kibona T, Halliday JEB, Thomas KM, Allan KJ, Jounson PCD, Davis A, Lankester F, Claxton JR, Rostal MK, Carter RW, de Jong RMF, Rubach MP, Crump JA, Mmbaga BT, Nyasebwa OM, Swai ES, Willett B, Cleaveland S (2022) Inter-epidemic Rift Valley fever virus infection incidence and risks for zoonotic spillover in northern Tanzania. PLoS Negl Trop Dis 16:e0010871
del Castillo SM, Bashaw MJ, Patton ML, Rieches RR, Bercovitch FB (2005) Fecal steroid analysis of female giraffe (Giraffa camelopardalis) reproductive condition and the impact of endocrine status on daily time budgets. Gen Comp Endocrinol 141:271–281
Doughty CE, Roman J, Faurby S, Wolf A, Haque A, Bakker ES, Malhi Y, Dunning JB Jr, Svenning J-C (2016) Global nutrient transport in a world of giants. Proc Natl Acad Sci 113:868–873
ESRI (2020) ArcGIS Desktop. Release 10.8.1. Redlands. Environmental Systems Research Institute, CA
Evans A, Gakuya F, Paweska JT, Rostal M, Akoolo L, Van Vuren PJ, Manyibe T, Macharia JM, Ksiazek TG, Feikin DR, Breiman RF, Kariuki Njenga M (2008) Prevalence of antibodies against Rift Valley fever virus in kenyan wildlife. Epidemiol Infect 136:1261–1269
Fennessey JT (2004) Foraging ecology. Chapter 7 in Ecology of desert-dwelling giraffe Giraffa camelopardalis angolensis in northwestern Namibia. PhD dissertation, University of Sydney, Sydney, Australia
Fischer EA, Boender GJ, Nodelijk G, Koeijer AA, van Roermund HJW (2013) The transmission potential of Rift Valley fever virus among livestock in the Netherlands: a modelling study. Vet Res 44:1–13
Foley C, Pettorelli N, Foley L (2008) Severe drought and calf survival in elephants. Biol Lett 4:541–544
Foster JB (1966) The giraffe of Nairobi National Park: home range, sex ratios, the herd, and food. Afr Wildl J 4:139–148
Fritz H, Duncan P, Gordon IJ, Illius AW (2002) Megaherbivores influence trophic guilds structure in african ungulate communities. Oecologia 131:620–625
Fryxell JM (1987) Food limitation and demography of a migratory antelope, the white-eared kob. Oecologia 72:83–91
Fuller A, Dawson T, Helmuth B, Hetem RS, Mitchell D, Maloney SK (2010) Physiological mechanisms in coping with climate changes. Physiol Biochem Zool: Ecol Evol Approach 83:713–720
Gaillard J-M, Boutin J-M, Delorme D, Van Laere G, Duncan P, Lebreton J-D (1997) Early survival in roe deer: causes and consequences of cohort variation in two contrasted populations. Oecologia 112:502–513
Galetti M, Moleón M, Jordano P, Pires MM, Guimarães PR Jr, Pape T, Nichols E, Hansen D, Olesen JM, Munk M, de Mattos JS, Schweiger AH, Owen-Smith N, Johnson CN, Marquis RJ, Svenning J-C (2018) Ecological and evolutionary legacy of megafauna extinctions. Biol Rev 93:845–862
Hampson K, Lembo T, Bessell P, Auty H, Packer C, Halliday J et al (2011) Predictability of anthrax infection in the Serengeti, Tanzania. J Appl Ecol 48:1333–1344
Hart EE, Fennessey J, Hauenstein S, Ciuti S (2020) Intensity of giraffe locomotor activity is shaped by solar and lunar zeitgebers. Behavi Processes 178:104178
Hiley P (1975) How the elephant keeps its cool. Nat Hist 84:34–40
Hopcraft JGC, Sinclair ARE, Packer C (2005) Planning for success; serengeti lions seek prey accessibility rather than abundance. J Anim Ecol 74:559–566
Huang Y-H, Kausrud K, Hassim A, Ochai SO, van Schalkwyk OL, Dekker EH et al (2022) Environmental drivers of biseasonal anthrax outbreak dynamics in two multihost savanna systems. Ecol Monogr 92:e1526
Isaac JL (2009) Effects of climate change on life history: implications for extinction risk in mammals. Endanger Species Res 7:115–123
Keesing F, Ostfeld RS, Young TP, Allan BF (2018) Cattle and rainfall affect tick abundance in central Kenya. Parasitology 145:345–354
Kiffner C, Peters L, Stroming A, Kioko J (2015) Bushmeat consumption in the Tarangire-Manyara ecosystem, Tanzania. Trop Conserv Sci 8:318–332
Knüsel MA, Lee DE, König B, Bond ML (2019) Correlates of home-range sizes of giraffes Giraffa camelopardalis. Anim Behav 149:143–151
Kuntsch V, Nel JAJ (1990) Possible thermoregulatory behaviour in Giraffa camelopardalis. Z Säugetierkd 55:60–62
Laake JL (2013) RMark: an R interface for analysis of capture-recapture data with MARK. AFSC Processed Rep 2013-01, 25p. Alaska Fish Sci Cent NOAA Natl Mar Fish Serv, 7600 Sand Point Way NE, Seattle WA 98115
Langman VA, Maloiy GMO, Schmidt-Nielsen K, Schroter RS (1979) Nasal heat exchange in a large mammal, the giraffe. Respir Physiol 37:325–333
Lee DE (2018) Evaluating conservation effectiveness in a Tanzanian Community Wildlife Management Area. J Wildl Manage
Lee DE, Bolger DT (2017) Movements and source-sink dynamics of a Masai giraffe metapopulation. Pop Ecol 59:57–168
Lee DE, Strauss MKL (2016) Giraffe demography and population ecology reference Module in Earth Systems and Environmental Science. Elsevier
Lee DE, Bond ML, Kissui BM, Kiwango YA, Bolger DT (2016a) Spatial variation in giraffe demography: a test of 2 paradigms. J Mammal 97:1015–1025
Lee DE, Kissui BM, Kiwango YA, Bond ML (2016b) Migratory herds of wildebeest and zebra indirectly affect juvenile survival of giraffes. Ecol Evol 6:8402–8411
Lee DE, Bond ML, Bolger DT (2017) Season of birth affects juvenile survival of giraffe. Pop Ecol 59:45–54
Lee DE, Fienieg e, Van Oosterhout C, Muller Z, Strauss M, Carter KD, Scheijen CPJ, Deacon F (2020) Giraffe translocation population viability analysis. Endanger Species Res 41:245–252
Levi M, Lee DE, Bond ML, Trydte AC (2022) Forage selection by Masai giraffes (Giraffa camelopardalis tippelskirchi) at multiple spatial scales. J Mammal 103:737–744
Lichtenfeld LL (2005) Our shared kingdom at risk: Human-lion relationships in the 21st century. PhD dissertation, Yale University
McSweeney C, New M, Lizcano G (2010) UNDP Climate Change Country Profiles: Tanzania. Available: http://country-profiles.geog.ox.ac.uk/ [Accessed 7 July 2016]
Milner JM, Nilsen EB, Andreassen HP (2007) Demographic side effects of selective hunting in ungulates and carnivores. Cons Biol 21:36–47
Mitchell G (2021) How giraffes work. Oxford University Press, USA
Mitchell G, Lust A (2008) The carotid rete and artiodactyl success. Biol Lett 4:415–418
Mitchell G, Skinner JD (2004) Giraffe thermoregulation: a review. Trans R Soc South Africa 59:49–57
Mitchell G, van Sittert S, Roberts D, Mitchell D (2017) Body surface area and thermoregulation in giraffes. J Arid Environ 145:35–42
Mkessa V (2022) Gastrointestinal parasite prevalence and intensity of Masai giraffes in Tarangire-Manyara Ecosystem. M.Sc. thesis, University of Glasgow, Glasgow, Scotland
Modafferi RD, Becker EF (1997) Survival of radiocollared adult moose in lower Susitna River Valley, Southcentral Alaska. J Wildl Manage 61:540–549
Muller Z (2018) Population structure of giraffes is affected by management in the Great Rift Valley. Kenya PlosONE 13:c0189678
Mumby HS, Courtiol A, Mar KU, Lummaa V (2013) Climatic variation and age-specific survival in asian elephants from Myanmar. Ecology 94:1131–1141
Mumby HS, Mar KU, Thitaram C, Courtiol A, Towiboon P, Min-Oo Z, Htut-Aung Y, Brown JL, Lummaa V (2015) Stress and body condition are associated with climate and demography in asian elephants. Conserv Physiol 3:cov030
Mwakapeje ER, Høgset S, Fyumagwa R, Nonga HE, Mdegela RH, Skerve E (2018) Anthrax outbreaks in the humans-livestock and wildlife interface areas of Northern Tanzania: a retrospective record review 2006–2016. BMC Public Health 18:106–127
Ogutu JO, Piepho H-P, Dublin HT, Bhola N, Reid RS (2008) Rainfall influences on ungulate population abundance in the Mara-Serengeti ecosystem. J Anim Ecol 77:814–829
Ogutu JO, Piepho H-P, Dublin HT (2013) Responses of phenology, synchrony and fecundity of breeding by african ungulates to interannual variation in rainfall. Wildl Res 40:698–717
Olff H, Ritchie ME, Prins HTH (2002) Global environmental controls of diversity in large herbivores. Nature 415:901–904
Owen-Smith N (1990) Demography of a large herbivore, the greater kudu Tragelaphus strepsiceros, in relation to rainfall. J Anim Ecol 59:893–913
Owen-Smith N (1992) Megaherbivores: the influence of very large body size on ecology. Cambridge University Press, Cambridge
Owen-Smith N, Mason DR, Ogutu JO (2005) Correlates of survival rates for 10 african ungulate populations: density, rainfall and predation. J Anim Ecol 74:774–788
Paniw M, Ozgul A, Salguero-Gómez R (2018) Interactive life-history traits predict sensitivity of plants and animals to temporal autocorrelation. Ecol Lett 21:275–286
Paniw M, James TD, Archer CR, Römer G, Levin S, Compagnoni A, Che-Castaldo J, Bennett JM, Mooney A, Childs ZC, Ozgul A, Jones OR, Burns JH, Beckerman AP, Patwary A, Sanchez-Gassen N, Knight TM, Salguero-Gómez R (2021) The myriad of complex demographic responses of terrestrial mammals to climate change and gaps of knowledge: a global analysis. J Anim Ecol 90:1398–1407
Paniw M, Duncan C, Groenewoud F, Drewe JA, Manser M, Ozgul A, Clutton-Brock T (2022) Higher temperature extremes exacerbate negative disease effects in a social mammal. Nat Clim Change 12:284–290
Pellew RA (1984) The feeding ecology of a selective feeder, the giraffe (Giraffa camelopardalis tippelskirchi). J Zool 292:57–81
Pollock KH (1982) A capture–recapture design robust to unequal probability of capture. J Wildl Manage 46:752–757
Pörtner HO (2022) In: Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, Craig M, Langsdorf S, Löschke S, Möller V, Okem A, Rama B (eds) Climate Change 2022: impacts, adaptation and vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK and New York, NY, USA, p 3056pp. IPCC
Pradel R (1996) Utilization of capture-mark for the study of recruitment and population growth. Biometrics 52:703–709
Pratt DJ, Greenway PJ, Gwynne MD (1966) A classification of east african rangeland, with an appendix on terminology. Br Ecol Soc 3:369–382
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rabaiotti D, Groom R, McNutt JW, Watermeyer J, O’Neill HMK, Woodroffe R (2021) High temperatures and human pressures interact to influence mortality in an African carnivore. Ecol Evol 11:8495–8506
Royle JA, Chandler RB, Sollmann R, Gardner B (2014) Spatial capture-recapture Academic Press. Elsevier, Waltham, MA, USA
Saether B-E, Coulson T, Grøtan V, Engen S, Altwegg R, Armitage KB, Barbraud C, Becker PH, Blumstein DT, Dobson FS, Festa-Bianchet M, Gaillard J-M, Jenkins A, Jones C, Nicoll MAC, Norris K, Oli MK, Ozgul A, Weimerskirch H (2013) How life history influences population dynamics in fluctuating environments. Am Nat 183:743–759
Sauer JR, Boyce MS (1983) Density dependence and survival of elk in northwestern Wyoming. J Wildl Manage 47:31–37
Shorrocks B (2016) The Giraffe: Biology, ecology, evolution and behaviour. Wiley-Blackwell, UK
Shrestha AK, van Wieren SE, van Langevelde F, Fuller A, Hetem RS, Meyer L, de Bie S, Prins HHT (2014) Larger antelopes are sensitive to heat stress throughout all seasons but smaller antelopes only during summer in an african semi-arid environment. Int J Biometeorol 58:41–49
Speakman JR, Król E (2010) Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J Anim Ecol 79:726–746
Strauss MKL, Packer C (2013) Using claw marks to study lion predation on giraffes of the Serengeti. J Zool 289:134–1423
Strauss MKL, Kilewo M, Rentsch D, Packer C (2015) Food supply and poaching limit giraffe abundance in the Serengeti. Pop Ecol 57:505–516
Suraud JP, Fennessy J, Bonnaud E, Issa AM, Fritz H, Gaillard J-M (2012) Higher than expected growth rate of the endangered west african giraffe Giraffa camelopardalis peralta: a successful human-wildlife cohabitation. Oryx 4:577–583
Titcomb G, Mantas JN, Hulke J, Rodriguez I, Branch D, Young H (2021) Water sources aggregate parasites with increasing effects in more arid conditions. Nat Comm 12:7066
Turner WC, Imologhome P, Havarua Z, Kaaya GP, Mfune JKE, Mpofu IDT, Getz WM (2013) Soil ingestion, nutrition and the seasonality of anthrax in herbivores of Etosha National Park. Ecosphere 4:13
VanderWaal K, Omondi GP, Obanda V (2014) Mixed-host aggregations and helminth parasite sharing in an east african wildlife–livestock system. Vet Parasitol 205:224–232
Watts HE, Holekamp KE (2009) Ecological determinants of survival and reproduction in the spotted hyena. J Mammal 90:461–471
Wensman JJ, Lindahl J, Wachtmeister N, Torsson E, Gwakisa P, Kasanga C, Misinzo G (2015) A study of Rift Valley fever virus in Morogoro and Arusha regions of Tanzania – serology and farmers’ perceptions. Infect Ecol Epidemiology 5:30025
Williams AE, Worsley-Tonks KEL, Ezenwa VO (2017) Drivers and consequences of variation in individual social connectivity. Anim Behav 133:1–9
Wittemyer G, Daballen D, Douglas-Hamilton I (2013) Comparative demography of an at-risk african elephant population. PLoS ONE 8:e53726
Woodroffe R, Groom R, McNutt JW (2017) Hot dogs: high ambient temperatures impact reproductive success in a tropical carnivore. J Anim Ecol 86:1329–1338
Acknowledgements
This study was conducted with permission from the Tanzania Commission for Science and Technology, Tanzania National Parks, Tanzania Wildlife Research Institute, Tanzania Wildlife Authority, African Wildlife Foundation, and Manyara Ranch Conservancy. MLB was supported by a Forschungskredit grant from the University of Zurich (FK-16-080), a Swiss National Science Foundation Postdoc.Mobility grant (P500PB_206670/1), and Parrotia-Stiftung. AO was supported by the Swiss National Science Foundation (Grant Number 31003A_182286). Field data collection funding was provided by the Sacramento Zoo, Tierpark Berlin, Tulsa Zoo, Columbus Zoo, The Living Desert, GreaterGood.org’s Project Peril, Save the Giraffes, Cincinnati Zoo, Zoo Miami, Como Park Zoo, Toronto Zoo, and World Giraffe Foundation.
Funding
Open access funding provided by University of Zurich
Author information
Authors and Affiliations
Contributions
MLB and DEL collected field data; MLB, DEL, and AO analyzed the data; MLB led the writing of the manuscript with input from DEL and AO.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest.
Additional information
Communicated by Roberto Cazzolla Gatti.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bond, M.L., Ozgul, A. & Lee, D.E. Effect of local climate anomalies on giraffe survival. Biodivers Conserv 32, 3179–3197 (2023). https://doi.org/10.1007/s10531-023-02645-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-023-02645-4