Abstract
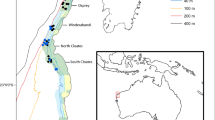
The transboundary networks of Marine Protected Areas (MPAs) project, TRANSMAP, assessed local turnover and regional biodiversity across the East African Marine Ecoregion, where inter-governmental co-operation has been working to connect local MPAs. The benthic fauna in the three most dominant habitats on this coastline—beaches, mangroves and seagrasses—were studied in two Regions (Northern Region, 10–13°S; Southern Region, 25–28°S). Meiofaunal taxa were used as the model faunal group owing to their diversity and abundance across habitat types and environmental conditions. Meiofaunal abundance averaged 2,500 individuals 10 cm−2 and was generally higher in mangrove and seagrass sediments than on the beaches, and was significantly different between habitats × Regions. In total, 18 taxa were recorded with highest diversity in the beach samples. Diversity indices and assemblage structure were significantly different between habitats, but also Regions. Specific granulometric 1Φ size classes, shore-height and number of rain days were the factors most significantly correlating with the observed assemblage patterns. Additionally, the size of a MPA and latitude (which correlated with MPA age, but not number of rain days), were the factors fitting best with meiofaunal assemblage patterns across the beaches, the habitat for which the most comprehensive data were generated. Sample diversity was higher in the Southern Region, and although within- and across-habitats diversity were similar across the Regions, the two Regions appeared to provide complementary habitats and supported different assemblages. Within the Regions, beaches (the only habitat for which more than one location was sampled) were significantly different between Locations, supporting the establishment of multiple protected locations of the same habitat within each transboundary MPA.





Similar content being viewed by others
Abbreviations
- °C:
-
Degrees centigrade
- μm:
-
Micrometres
- ANOSIM:
-
Analysis of similarity
- ANOVA:
-
Analysis of variance
- BDI:
-
Beach Deposit Index
- BI:
-
Beach Index
- EAME:
-
East African Marine Ecoregion
- EC:
-
European Commission
- GPS:
-
Global Positioning System
- HW:
-
High water
- INCO-DEV:
-
International Co-operation for Development
- km:
-
Kilometres
- L:
-
Lower-shore
- LW:
-
Low water
- m:
-
Metre
- M:
-
Mid-shore
- MHWN:
-
Mean high water neap
- MHWS:
-
Mean high water spring
- MLWN:
-
Mean low water neap
- MLWS:
-
Mean low water spring
- mm:
-
Millimetres
- MPA:
-
Marine Protected Area
- NHM:
-
Natural History Museum, London
- PERMANOVA:
-
Permutational multivariate analysis of variance
- PRIMER:
-
Plymouth routines in multivariate ecological research
- TRANSMAP:
-
Transboundary networks of Marine Protected Areas for integrated conservation and sustainable development: biophysical, socio-economic and governance assessment in East Africa
- U:
-
Upper-shore
- WWF:
-
World Wide Fund for Nature
- y/n:
-
Yes/no
References
Albuquerque EF, Pinto APB, de Queiroz Perez AA, Veloso VG (2007) Spatial and temporal changes in interstitial meiofauna on a sandy ocean beach of South America. Braz J Oceanogr 55(2):121–131
Alongi DM (1987a) Intertidal zonation and seasonality of meiobenthos in tropical mangrove estuaries. Mar Biol 95(3):447–458
Alongi DM (1987b) The influence of mangrove-derived tannins on intertidal meiobenthos in tropical estuaries. Oecologia 71(4):537–540
Alongi DM, Christoffersen P (1992) Benthic infauna and organism–sediment relations in a shallow, tropical coastal area—influence of outwelled mangrove detritus and physical disturbance. Mar Ecol Prog Ser 81(3):229–245
Alongi DM, Boto KG, Tirendi F (1989) Effect of exported mangrove litter on bacterial productivity and dissolved organic-carbon fluxes in adjacent tropical nearshore sediments. Mar Ecol Prog Ser 56(1–2):133–144
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Ansari ZA (1984) Benthic macro and meiofauna of seagrass (Thalassia-hemprichii) bed at, Minicoy, Lakshadweep. Indian J Mar Sci 13(3):126–127
Ansari ZA, Parulekar AH (1994) Meiobenthos in the sediments of seagrass meadows of Lakshadweep Atolls, Arabian Sea. Vie Milieu 44(3/4):185–190
Ansari ZA, Ramani P, Rivonker CU, Parulekar AH (1990) Macro and meiofaunal abundance in six sandy beaches of Lakshadweep islands. Indian J Mar Sci 19:159–164
Armonies W, Reise K (2000) Faunal diversity across a sandy shore. Mar Ecol Prog Ser 196:49–57
Attrill MJ (2002) A testable linear model for diversity trends in estuaries. J Anim Ecol 71:262–269
Barnes N, Bamber RN, Moncrieff CB, Sheader M, Ferrero TJ (2008) Meiofauna in closed coastal saline lagoons in the United Kingdom: structure and biodiversity of the nematode assemblage. Estuar Coast Shelf Sci 79(2):328–340
Boaden PJS (1968) Water movement: a dominant factor in interstitial ecology. Sarsia 34:125–136
Boström C, Jackson EL, Simenstad CA (2006) Seagrass landscapes and their effects on associated fauna: a review. Estuar Coast Shelf Sci 68:383–403
Buchanan JB (1984) Sediment analysis. In: Holmes NA, McIntyre AD (eds) Methods for the study of marine benthos. Blackwell Scientific Publications, Oxford
Chinnadurai G, Fernando OJ (2003) Meiofauna of Pichavaram mangroves along southeast coast of India. J Mar Biol Assoc India 45(2):158–165
Chinnadurai G, Fernando OJ (2006) Meiobenthos of Cochin mangroves (Southwest coast of India) with emphasis on free-living marine nematode assemblages. Russ J Nematol 14(2):127–137
Clarke KR, Gorley RN (2001) PRIMER v5: user manual/tutorial. PRIMER-E, Plymouth. http://web.pml.ac.uk/primer/index.html. Accessed 10 June 2009
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Plymouth. ISBN 1855311402
Coull BC (1999) Role of meiofauna in estuarine soft-bottom habitats. Aust J Ecol 24(4):327–343
Danovaro R (1996) Detritus–bacteria–meiofauna interactions in a seagrass bed (Posidonia oceanica) of the NW Mediterranean. Mar Biol 127(1):1–13
Day JW, Coronado Molina C, Vera Herrera FR, Twilley R, Rivera Monroy VH, Alvarez Guillen H, Day R, Conner W (1996) A 7 year record of above-ground net primary production in a southeastern Mexican mangrove forest. Aquat Bot 55(1):39–60
de Boer WF (2000) Biomass dynamics of seagrasses and the role of mangrove and seagrass vegetation as different nutrient sources for an intertidal ecosystem. Aquat Bot 66(3):225–239
de Boer WF, Prins HHT (2002) Human exploitation and benthic community structure on a tropical intertidal flat. J Sea Res 48(3):225–240
de Jonge VN, Bouwman LA (1977) A simple density separation technique for quantitative isolation of meiobenthos using the colloidal silica gel Ludox T.M. Mar Biol 42:143–148
de la Huz R, Lastra M (2008) Effects of morphodynamic state on macrofauna community of exposed sandy beaches of Galician coast. PSZNI Mar Ecol 29(Suppl 1):150–159
de la Torre-Castro M, Ronnback P (2004) Links between humans and seagrasses—an example from tropical East, Africa. Ocean Coast Manag 47(7–8):361–387
De Troch M, Gurdebeke S, Fiers F, Vincx M (2001) Zonation and structuring factors of meiofauna communities in a tropical seagrass bed (Gazi Bay, Kenya). J Sea Res 45(1):45–61
De Troch M, Van Gansbeke D, Vincx M (2006) Resource availability and meiofauna in sediment of tropical seagrass beds: local versus global trends. Mar Environ Res 61(1):59–73
Decho AW, Hummon WD, Fleeger JW (1985) Meiofauna–sediment interactions around sub-tropical seagrass sediments using factor-analysis. J Mar Res 43(1):237–255
Defeo O, McLachlan A, Schoeman DS, Schlacher TA, Dugan J, Jones A, Lastra M, Scapini F (2009) Threats to sandy beach ecosystems: a review. Estuar Coast Shelf Sci 81:1–12
Dye AH (1983) Composition and seasonal fluctuations of meiofauna in a Southern African mangrove estuary. Mar Biol 73:165–170
Dye AH (2006) Persistent effects of physical disturbance on meiobenthos in mangrove sediments. Mar Environ Res 62(5):341–355
Dye AH, McLachlan A, Wooldridge T (1981) The ecology of sandy beaches in Natal. S Afr J Zool 16:200–209
Edgar GJ (1999) Experimental analysis of structural versus trophic importance of seagrass beds. I. Effects on macrofaunal and meiofaunal invertebrates. Vie Milieu 49(4):239–248
Edgar GJ, Shaw C, Watson GF, Hammond LS (1994) Comparisons of species richness, size-structure and production of benthos in vegetated and unvegetated habitats in Western-Port, Victoria. J Exp Mar Biol Ecol 176(2):201–226
Elmgren R (1973) Methods of sampling sublittoral soft bottom meiofauna. Oikos 15(Suppl):112–120
Ewel KC, Cressa C, Kneib RT, Lake PS, Levin LA, Palmer MA, Snelgrove P, Wall DH (2001) Managing critical transition zones. Ecosystems 4(5):452–460
Ferrero TJ, Debenham NJ, Lambshead PJD (2008) The nematodes of the Thames estuary: assemblage structure and biodiversity, with a test of Attrill’s linear model. Estuar Coast Shelf Sci 79(3):409–418
Gee JM, Somerfield PJ (1997) Do mangrove diversity and leaf litter decay promote meiofaunal diversity? J Exp Mar Biol Ecol 218(1):13–33
Giere O (1993) Meiobenthology. The microscopic fauna in aquatic sediments. Springer, Berlin
Gomes CAA, Santos PJP, Alves TNC, Rosa-Filho JS, Souza-Santos LP (2002) Variação temporal da meiofauna em área de manguezal em Itamaracá-Pernambuco. Atlântica 24:89–96
Gourbault N, Warwick RM, Helleouet MN (1998) Spatial and temporal variability in the composition and structure of meiobenthic assemblages (especially nematodes) in tropical beaches (Guadeloupe, FWI). Cah Biol Mar 39(1):29–39
Gullstrom M, de la Torre Castro M, Bandeira SO, Bjork M, Dahlberg M, Kautsky N, Ronnback P, Ohman MC (2002) Seagrass ecosystems in the Western Indian Ocean. Ambio 31(7–8):588–596
Harris RP (1972) The distribution and ecology of the interstitial meiofauna of a sandy beach at Whitsand Bay, east Cornwall. J Mar Biol Assoc UK 52:1–18
Hemminga MA, Slim FJ, Kazungu J, Ganssen GM, Nieuwenhuize J, Kruyt NM (1994) Carbon outwelling from a mangrove forest with adjacent seagrass beds and coral-reefs (Gazi Bay, Kenya). Mar Ecol Prog Ser 106(3):291–301
Higgins RP, Thiel H (1988) Introduction to the study of meiofauna. Smithsonian Institution Press, Washington, DC
Hillebrand H (2004) Strength, slope and variability of marine latitudinal gradients. Mar Ecol Prog Ser 273:251–267
Hooge MD (1999) Abundance and horizontal distribution of meiofauna on a Northern California beach. Pac Sci 53(3):305–315
Hulings NC (1974) A temporal study of Lebanese sand beach meiofauna. Cah Biol Mar 15:319–335
Kalk M (1995) A natural history of Inhaca Island. Witwatersrand University Press, Johannesburg
Kemp J (2000) WWF—East African marine ecoregion: biological reconnaissance final report. http://eame.wiomsa.org/pubs/EAME-Bio-Recce.pdf. Accessed 19 Feb 2008
Kotta J, Boucher G (2001) Interregional variation of free-living nematode assemblages in tropical coral sands. Cah Biol Mar 42(4):315–326
Kotwicki L, Szymelfenig M, De Troch M, Urban-Malinga B, Weslawski JM (2005) Latitudinal biodiversity patterns of meiofauna from sandy littoral beaches. Biodivers Conserv 14:461–474
Kristensen E, Bouillon S, Dittmar T, Marchand C (2008) Organic carbon dynamics in mangrove ecosystems: a review. Aquat Bot 89(2):201–219
Lambshead PJD, Brown CJ, Ferrero TJ, Mitchell NJ, Smith CR, Hawkins LE, Tietjen J (2002) Latitudinal diversity patterns of deep-sea marine nematodes and organic fluxes: a test from the central equatorial Pacific. Mar Ecol Prog Ser 236:129–135
Levin LA, Boesch DF, Covich A, Dahm C, Erséus C, Ewel KC, Kneib RT, Moldenke A, Palmer MA, Snelgrove P, Strayer D, Weslawski JM (2001) The function of marine critical transition zones and the importance of sediment biodiversity. Ecosystems 4:430–451
Lutjeharms JRE (2006) The ocean environment off southeastern Africa: a review. S Afr J Sci 102(9–10):419–426
Mackey AP, Smail G (1995) Spatial and temporal variation in litter fall of Avicennia-marina (Forssk) Vierh in the Brisbane-River, Queensland, Australia. Aquat Bot 52(1–2):133–142
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance based redundancy analysis. Ecology 82:290–297
McClanahan TR (1988) Seasonality in east Africa’s coastal waters. Mar Ecol Prog Ser 44(2):191–199
McKee KL, Faulkner PL (2000) Restoration of biogeochemical function in mangrove forests. Restor Ecol 8(3):247–259
McLachlan A (1977a) Studies on the psammolittoral meiofauna of Algoa Bay. II. The distribution, composition and biomass of the meiofauna and macrofauna. Zool Afr 12(1):33–60
McLachlan A (1977b) Composition, distribution, abundance and biomass of the macrofauna and meiofauna of four sandy beaches. Zool Afr 12(2):279–306
McLachlan A, Dorvlo A (2005) Global patterns in sandy beach macrobenthic communities. J Coast Res 21(4):674–687
McLachlan A, Dorvlo A (2007) Species–area relationships for sandy beach macrobenthos in the context of intertidal width. Oceanologia 49(1):91–98
McLachlan A, Jaramillo E (1995) Zonation on sandy beaches. Oceanogr Mar Biol 33:305–335
McLachlan A, Erasmus T, Furstenberg JP (1977) Migrations of sandy beach meiofauna. Zool Afr 12(2):257–277
McLachlan A, Wooldridge T, Dye AH (1981) The ecology of sandy beaches in Southern-Africa. S Afr J Zool 16(4):219–231
McLachlan A, de Ruyck A, Hacking N (1996) Community structure on sandy beaches: patterns of richness and zonation in relation to tide range and latitude. Rev Chil Hist Nat 69:451–467
Monthum Y, Aryuthaka C (2006) Spatial distribution of meiobenthic community in the Tha Len seagrass bed, Krabi Province, Thailand. Coast Mar Sci 30(1):146–153
Moore HB (1972) Aspects of stress in the tropical marine environment. Adv Mar Biol 10:217–269
Moreno M, Granelli V, Albertelli G, Fabiano M (2005) Meiofaunal distribution in microtidal mixed beaches of the Ligurian Sea (NW Mediterranean). Meiofauna Mar 14:131–137
Moreno M, Ferrero TJ, Granelli V, Marin V, Albertelli G, Fabiano M (2006) Across shore variability and trophodynamic features of meiofauna in a microtidal beach of the NW Mediterranean. Estuar Coast Shelf Sci 66(3–4):357–367
Moreno M, Ferrero TJ, Gallizia I, Vezzulli L, Albertelli G, Fabiano M (2008) An assessment of the spatial heterogeneity of environmental disturbance within an enclosed harbour through the analysis of meiofauna and nematode assemblages. Estuar Coast Shelf Sci 77(4):565–576
Nagelkerken I, Blaber SJM, Bouillon S, Green P, Haywood M, Kirton LG, Meynecke JO, Pawlik J, Penrose HM, Sasekumar A, Somerfield PJ (2008) The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat Bot 89(2):155–185
Ndaro SGM, Sjoling S, Olafsson E (1995) Small-scale variation in major meiofaunal taxa and sediment chemistry in tropical sediments. Ambio 24(7–8):470–474
Netto SA, Gallucci F (2003) Meiofauna and macrofauna communities in a mangrove from the Island of Santa Catarina, South Brazil. Hydrobiologia 505(1–3):159–170
Nicholas WL (2001) Seasonal variations in nematode assemblages on an Australian ocean beach; the effect of heavy seas and unusually high tides. Hydrobiologia 464:17–26
Nybakken JW (1997) Marine Biology. An ecological approach, 4th edn. Addison-Wesley Educational Publications Inc, Reading
Olafsson E (1995) Meiobenthos in mangrove areas in eastern Africa with emphasis on assemblage structure of free-living marine nematodes. Hydrobiologia 312(1):47–57
Olafsson E, Carlstrom S, Ndaro SGM (2000) Meiobenthos of hypersaline tropical mangrove sediment in relation to spring tide inundation. Hydrobiologia 426(1–3):57–64
Olafsson E, Buchmayer S, Skov MW (2002) The East African decapod crab Neosarmatium meinerti (de Man) sweeps mangrove floors clean of leaf litter. Ambio 31(7–8):569–573
Pattnaik A, Rao L (1990) Composition and distribution of interstitial meiofauna of the sandy beach at Gopalpur, south Orissa coast. Indian J Mar Sci 19:165–170
Paula J, Costa PFE, Martins A, Gove D (2001) Patterns of abundance of seagrasses and associated infaunal communities at Inhaca Island, Mozambique. Estuar Coast Shelf Sci 53(3):307–318
Pinto TKDO, Santos PJPD (2006) Meiofauna community structure variability in a Brazilian tropical sandy beach. Atlântica 28(2):117–127
Platt HM, Warwick RM (1988) Free-living marine nematodes. Part II. British Chromadorida. Synopses of the British Fauna no. 38. Brill, Leiden
Quartly GD, Srokosz MA (2003) Satellite observations of the Agulhas Current system. Philos Trans R Soc A 361(1802):51–56
Renaud-Mornant J, Gourbault N (1984) First meiofaunistic survey of Guadeloupe. Hydrobiologia 118(1):113–118
Roberts MJ, Ribbink AJ, Morris T, van den Berg MA, Engelbrecht DC, Harding RT (2006) Oceanographic environment of the Sodwana Bay coelacanths (Latimeria chalumnae), South Africa. S Afr J Sci 102:435–443
Rodriguez JG, Lopez J, Jaramillo E (2001) Community structure of the intertidal meiofauna along a gradient of morphodynamic sandy beach types in southern Chile. Rev Chil Hist Nat 74(4):885–897
Sasekumar A (1994) Meiofauna of a mangrove shore on the West-Coast of Peninsular Malaysia. Raffles B Zool 42(4):901–915
Schlacher TA, Schoeman DS, Dugan J, Lastra M, Jones A, Scapini F, McLachlan A (2008) Sandy beach ecosystems: key features, sampling issues, management, challenges and climate change impacts. Mar Ecol Evol Perspect 29(Suppl 1):70–90
Schrijvers J, Okondo J, Steyaert M, Vincx M (1995) Influence of epibenthos on meiobenthos of the Ceriops tagal mangrove sediment at Gazi Bay Kenya. Mar Ecol Prog Ser 128:247–259
Sheridan P (1997) Benthos of adjacent mangrove, seagrass and non-vegetated habitats in Rookery Bay, Florida, USA. Estuar Coast Shelf Sci 44(4):455–469
Siebert T, Branch GM (2007) Influences of biological interactions on community structure within seagrass beds and sandprawn-dominated sandflats. J Exp Mar Biol Ecol 340(1):11–24
Soares G (2003) Sandy beach morphodynamics and macrobenthic communities in temperate, subtropical and tropical regions—a macroecological approach. Dissertation, University of Port Elizabeth, South Africa
Soetaert K, Vincx M, Wittoeck J, Tulkens M (1995) Meiobenthic distribution and nematode community structure in 5 European estuaries. Hydrobiologia 311(1–3):185–206
Somerfield PJ, Gee JM, Aryuthaka C (1998) Meiofaunal communities in a Malaysian mangrove forest. J Mar Biol Assoc UK 78(3):717–732
Steyaert M, Herman PMJ, Moens T, Widdows J, Vincx M (2001) Tidal migration of nematodes on an estuarine tidal flat (the Molenplaat, Schelde Estuary, SW Netherlands). Mar Ecol Prog Ser 224:299–304
Urban-Malinga B, Kotwicki L, Gheskiere TLA, Jankowska K, Opalinski K, Malinga M (2004) Composition and distribution of meiofauna, including nematode genera, in two contrasting Arctic beaches. Polar Biol 27:447–457
Vanaverbeke J, Vincx M (2008) Short-term changes in nematode communities from an abandoned intense sand extraction site on the Kwintebank (Belgian Continental Shelf) two years post-cessation. Mar Environ Res 66(2):240–248
Vanhove S, Vincx M, Vangansbeke D, Gijselinck W, Schram D (1992) The meiobenthos of 5 mangrove vegetation types in Gazi Bay, Kenya. Hydrobiologia 247(1–3):99–108
Warwick RM, Clarke KR (1993) Increased variability as a symptom of stress in marine communities. J Exp Mar Biol Ecol 172(1–2):215–226
Wells S, Ngusaru A (2004) WWF Eastern African marine ecoregion. Towards the establishment of an ecologically representative network of marine protected areas in Kenya, Tanzania and Mozambique. WWF, Dar es Salaam. http://eame.wiomsa.org/pubs/EAME-MPA-Network.pdf. Accessed 19 Feb 2008
Wieser W (1959) The effect of grain size on the distribution of small invertebrates inhabiting the beaches of Puget Sound. Limnol Oceanogr 4:181–194
Acknowledgments
We are grateful to the EC under the Sixth Framework programme, Specific Measures in Support of International Co-operation (INCO-DEV)—PRIORITY A2.2. Reconciling multiple demands on coastal zones, for funding the sampling, meiofaunal extraction and identification for this study. It forms part of the larger EC TRANSMAP project (http://www.transmap.fc.ul.pt/), Transboundary networks of marine protected areas for integrated conservation and sustainable development: biophysical, socio-economic and governance assessment in East Africa (Contract no.: PL 510862), co-ordinated by José Pavão Mendes de Paula of the Fundação da Universidade de Lisboa. We would like to thank Ricardo Nogueira Mendes from the Faculdade de Ciências da Universidade de Lisboa for his help with the Fig. 1. NB and TJF would also like to thank Dr Gordon Paterson, NHM, for critically reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barnes, N., Bamber, R.N., Bennell, G. et al. Assessment of regional and local biodiversity in tropical and subtropical coastal habitats in the East African Marine Ecoregion. Biodivers Conserv 20, 2075–2109 (2011). https://doi.org/10.1007/s10531-011-0076-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-011-0076-2