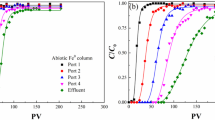
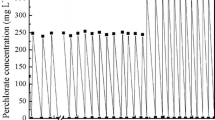
Abstract
Perchlorate was reduced by a mixed bacterial culture over a pH range of 7.0–8.9. Similar rates of perchlorate reduction were observed between pH 7.0 and 8.5, whereas significantly slower reduction occurred at pH 8.9. Addition of iron metal, Fe(0), to the mixed bacterial culture resulted in slower rates of perchlorate reduction. Negligible perchlorate reduction was observed under abiotic conditions with Fe(0) alone in a reduced anaerobic medium. The inhibition of perchlorate reduction observed in the presence of Fe(0) is in contrast to previous studies that have shown faster rates of contaminant reduction when bacteria and Fe(0) were combined compared to bacteria alone. The addition of Fe(0) resulted in a rise in pH, as well as precipitation of Fe minerals that appeared to encapsulate the bacterial cells. In experiments where pH was kept constant, the addition of Fe(0) still resulted in slower rates of perchlorate reduction suggesting that encapsulation of bacteria by Fe precipitates contributed to the inhibition of the bacterial activity independent of the effect of pH on bacteria. These results provide the first evidence linking accumulation of iron precipitates at the cell surface to inhibition of environmental contaminant degradation. Fe(0) was not a suitable amendment to stimulate perchlorate-degrading bacteria and the bacterial inhibition caused by precipitation of reduced Fe species may be important in other combined anaerobic bacterial–Fe(0) systems. Furthermore, the inhibition of bacterial activity by iron precipitation may have significant implications for the design of in situ bioremediation technologies for treatment of perchlorate plumes.
Similar content being viewed by others
References
Alowitz MJ & Scherer MM (2002) Kinetics of nitrate, nitrite, and Cr(VI) reduction by iron metal. Environ. Sci. Technol. 36: 299–306
Andrews EJ & Novak PJ (2001) Influence of ferrous iron and ph on carbon tetrachloride degradation by Methanosarcina thermophila. Water Res. 35: 2307–2313
APHA, AWWA & WEF (1989) Standard Methods for Examination of Water and Wastewater
Attaway H & Smith M (1993) Reduction of perchlorate by an anaerobic enrichment culture. J. Indust. Microbiol. 12: 408–412
Bruce RA, Achenbach LA & Coates JD (1999) Reduction of (per) chlorate by a novel organism isolated from paper mill waste. Environ. Microbiol. 1: 319–329
Coates JD, Michaelidou U, Bruce RA, O'Connor SM, Crespi JN & Achenbach LA (1999) Ubiquity and diversity of dissimilatory (per) chlorate-reducing bacteria. Appl. Environ. Microbiol. 65: 5234–5241
Daniels L, Belay N, Rajagopal BS & Weimer PJ (1987) Bacterial methanogenesis and growth from CO2 with elemental iron as the sole source of electrons. Science 237: 509–511
EPA, US (2002) Perchlorate. http: //www. epa. gov/safewater/ ccl/perchlor/perchlo. Html February 19, 2002
Gillham RW & Ohannesin SF (1994) Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water 32: 958–967
Glasauer S, Langley S & Beveridge TJ (2001) Sorption of Fe (hydr) oxides to the surface of Shewanella putrefaciens: cell-bound ne-grained minerals are not always formed de novo. Appl. Environ. Microbiol. 67: 5544–5550
Gregory KB, Mason MG, HD Picken, Weathers LJ & Parkin GF (2000) Bioaugmentation of Fe(0) for the remediation of chlorinated aliphatic hydrocarbons. Environ. Eng. Sci. 17: 169–181
Gurol M & Kim K (2000) Investigation of perchlorate removal in drinking water sources by chemical methods. In: Urbansky ET (Ed) Perchlorate in the Environment. Kluwer Academic Publishers/Plenum, New York
Herman DC & Frankenberger WT (1998) Microbial-mediated reduction of perchlorate in groundwater. J. Environ. Qual. 27: 750–754
Kersters I & Verstraete W (1996) Inactivation of aeromonas hydrophila by Fe(II)-related-radical generation in oxidizing groundwaters. Appl. Environ. Microbiol. 62: 3277–3283
Kim S & Picardal FW (1999) Enhanced anaerobic biotrans-formation of carbon tetrachloride in the presence of reduced iron oxides. Environ. Toxicol. Chem. 18: 2142–2150
Klausen J, Troeber SP, Haderlein SB & Schwarzenbach RP (1995) Reduction of substituted nitrobenzenes by Fe(II) in aqueous mineral suspensions. Environ. Sci. Technol. 29: 2396–2404
Liu C, Zachara JM, YA Gorby, Szecsody JE & Brown CF (2001) Microbial reduction of Fe(III) and sorption/precipitation of Fe(II) on Shewanella putrefaciens strain CN32. Environ. Sci. Technol. 35: 1385–1393
Madigan MT, Martinko JM & Parker J (1996) Brock Biology of Microorganisms, 8th edn. Prentice-Hall, Upper Saddle River, NJ
Matheson LJ & Tratnyek PG (1994) Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 28: 2045–2053
McCormick ML, Bouwer EJ & Adriaens P (2002) Carbon tetrachloride transformation in a model iron-reducing cul-ture: relative kinetics of biotic and abiotic reactions. Environ. Sci. Technol. 36: 403–410
Moore AM, De Leon CH & Young TM (2003) Rate and extent of aqueous perchlorate removal by iron surfaces. Environ. Sci. Technol. 37: 3189–3198
Novak PJ, Daniels L & Parkin GF (1998) Enhanced dechlorination of carbon tetrachloride and chloroform in the presence of elemental iron and Methanosarcina barkeri, 31. Methanosarcina thermophila, or Methanosaeta concillii. Environ. Sci. Technol. 32: 1438–1443
Rajagopal BS & Legall J (1989) Utilization of cathodic hydrogen by hydrogen-oxidizing bacteria. Appl. Microbiol. Biotechnol. 31: 406–412
Ram MS, Singh L, Suryanarayana MVS & Alam SI (2000) Effect of iron, nickel and cobalt on bacterial activity and dynamics during anaerobic oxidation of organic matter. Water Air Soil Pollut. 117: 305–312
Rancourt DG & Ping JY (1991) Voigt-based methods for arbitrary-shape static hyper ne parameter distributions in Mo ¨ssbauer-spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms 58: 85–97
Rikken GB, Kroon AGM & vanGinkel CG (1996) Transformation of (per) chlorate into chloride by a newly isolated bacterium: reduction and dismutation. Appl. Microbiol. Biotechnol. 45: 420–426
Roden EE & Urrutia MM (2002) Influence of biogenic Fe(II) on bacterial crystalline Fe(III) oxide reduction. Geomicrobiol. J. 19: 209–251
Sawyer CN, McCarty PL & Parkin GF (2003) Chemistry for Environmental Engineering 5th edn. McGraw Hill, New York
Shrout JD (2002) Characteristics and electron donor require-ments of perchlorate degradation by mixed and pure-culture bacteria. Ph. D. Dissertation. Iowa City, Iowa, The University of Iowa
Shrout JD & Parkin GF (2000) Inhibition of anaerobic perchlorate biotransformation by Fe(0) In Proceedings of 2nd International Conference on Remediation of Chlorinated and Recalcitrant Compounds, May 22–25, 2000, Battelle Press, Monterey, CA
Till BA, Weathers LJ & Alvarez PJ (1998) Fe(0)-supported autotrophic denitri cation. Environ. Sci. Technol. 32: 634–639
Urbansky ET (1998) Perchlorate chemistry: implications for analysis and remediation. Bioremed. J. 2: 81–95
vanGinkel CG, Plugge CM & Stroo CA (1995) Reduction of chlorate with various energy substrates and inocula under anaerobic conditions. Chemosphere 31: 4057–4066
Wallace W, Ward T, Breen A & Attaway H (1996) Identification of an anaerobic bacterium which reduces perchlorate and chlorate as Wolinella succinogenes. J. Indust. Microbiol. 16: 68–72
Warren LA & Ferris FG (1998) Continuum between sorption and precipitation of Fe(III) on microbial surfaces. Environ. Sci. Technol. 32: 2331–2337
Weathers LJ, Parkin GF & Alvarez PJ (1997) Utilization of cathodic hydrogen as electron donor for chloroform come-tabolism by a mixed, methanogenic culture. Environ. Sci. Technol. 31: 880–885
Weber KA, Picardal FW & Roden EE (2001) Microbially catalyzed nitrate-dependent oxidation of biogenic solid-phase Fe(II) compounds. Environ. Sci. Technol. 35: 1644–1650
Zhang HS, Bruns MA & Logan BE (2002) Perchlorate reduction by a novel chemolithoautotrophic, hydrogen-oxi-dizing bacterium. Environ. Microbiol. 4: 570–576
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shrout, J.D., Williams, A.G., Scherer, M.M. et al. Inhibition of bacterial perchlorate reduction by zero-valent iron. Biodegradation 16, 23–32 (2005). https://doi.org/10.1007/s10531-004-0354-3
Issue Date:
DOI: https://doi.org/10.1007/s10531-004-0354-3