Abstract
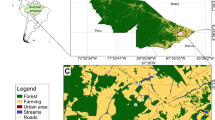
Peacock basses (Cichla spp.) are native to the Amazon basin but introduced to different parts of the world. Almost thirty years ago, Cichla kelberi was introduced in an impoundment of the São João River, a coastal system in southeastern Brazil. Recently, this cichlid invaded the estuarine section of the basin. This study aims to analyze spatial and temporal variations in catch of C. kelberi and fish assemblage structure along the estuarine stretch of this river and how abiotic variables affect their distribution. Sampling was performed in four segments downstream of the dam. Principal component analysis revealed that abiotic variables displayed temporal and spatial variation, in part due to the salinity gradient, that were more pronounced in the dry season. Cichla kelberi occurred in all segments, but mainly in shallow and vegetated habitats of the middle course and barely in the most downstream. Eighty-one fish species were recorded, nine of which were non-native, representing 33.4% of total catch. A redundancy analysis indicated that the fish assemblages showed marked spatial variation, mostly related to the salinity gradient. The lowermost segment of the river was dominated by marine species, the only locality where non-native species summed less than 40% of the catches. In the upstream segments, higher oxygen levels and lower temperatures influenced the occurrence of most species. Higher salinity of the estuary seems to limit the spread of C. kelberi, but the invader may reach adjacent basins in years of exceptional floods. The eurihalinity and piscivory of C. kelberi partly explain its invasive success.






Similar content being viewed by others
References
Agostinho A, Thomaz SM, Gomes LC (2005) Conservation of the biodiversity of Brazil’s inland waters. Conserv Biol 19(3):646–652
Agostinho AA, Gomes LC, Pelicice FM (2007) Ecologia e manejo de recursos pesqueiros em reservatórios do Brasil. Eduem, Maringá
Agostinho AA, Pelicice FM, Gomes LC (2008) Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz J Biol 68(4):1119–1132
Alber MA (2002) A conceptual model of estuarine freshwater inflow management. Estuaries 25(6B):1246–1261
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Z 22(6):711–728. https://doi.org/10.1127/0941-2948/2013/0507
Andrade-Tubino MF, Ribeiro ALR, Vianna M (2008) Organização espaço-temporal das ictiocenoses demersais nos ecossistemas estuarinos brasileiros: uma síntese. Oecolog Bras 12(4):640–661
Assis DAS, Dias-Filho VA, Magalhães ALB, Brito MFG (2017) Establishment of the non-native fish Metynnis lippincottianus (Cope 1870) (Characiformes: Serrasalmidae) in lower São Francisco River, northeastern Brazil. Stud Neotrop Fauna E 52(3):2–11
Barletta M, Barletta-Bergan AB, Saint-Paul U, Hubold G (2005) The role of salinity in structuring the fish assemblages in a tropical estuary. J Fish Biol 66:45–72. https://doi.org/10.1590/S1679-62252012000100011
Beatty SJ, Morgan DL (2013) Introduced freshwater fishes in a global endemic hotspot and implications of habitat and climatic change. BioInvasions Rec 2(1):1–9
Bizerril CSRF (1995) Análise da distribuição espacial da ictiofauna de uma bacia hidrográfica do Leste brasileiro. Arq Biol Tecnol 38(2):477–499
Blaber SJM (2000) Tropical estuarine fishes. Ecology, exploitation and conservation. Blackwell Science, Oxford
Blanchet FG, Legendre P, Borcard D (2008) Forward selection of explanatory variables. Ecology 89:2623–2632
Brito MFG, Magalhães ALB (2017) Brazil’s development turns river into sea. Science 358:179
Corbacho C, Sanchéz JM (2001) Patterns of species richness and introduced species in native freshwater fish faunas of a Mediterrenean-type Basin: the Guadiana River (southwest Iberian Peninsula). Regul Rivers Res Manag 17:699–707. https://doi.org/10.1002/rrr.631
Daga VD, Skóra F, Padial AA, Abilhoa V, Gubiani EA, Vitule JRS (2015) Homogenization dynamics of the fish assemblages in Neotropical reservoirs: comparing the roles of introduced species and their vectors. Hydrobiologia 746:327–347. https://doi.org/10.1007/s10750-014-2032-0
Elliot M, Whitfield AK, Potter IC, Blaber SJM, Cyrus DP, Nordlie FG, Harrison TD (2007) The guild approach to categorizing estuarine fish assemblages: a global review. Fish Fish 8:241–268
Eschmeyer WN, Fricke R (2016) Catalog of fishes: genera, species, references. https://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp). Electronic version Accessed 12 Feb 2016
Espínola LA, Minte-Vera CV, Júlio HF Jr (2010) Invasibility of reservoirs in the Paraná Basin, Brazil, to Cichla kelberi Kullander and Ferreira 2006. Biol Invas 12:1889–1889
Figueiredo JL, Menezes NA (1978) Manual de Peixes Marinhos do Sudeste do Brasil. II. Teleostei (1). Museu de Zoologia da Universidade de São Paulo, São Paulo
Figueiredo JL, Menezes NA (1980) Manual de Peixes Marinhos do Sudeste do Brasil. III. Teleostei (2). Museu de Zoologia da Universidade de São Paulo, São Paulo
Figueiredo JL, Menezes NA (2000) Manual de Peixes Marinhos do Sudeste do Brasil. VI. Teleostei (5) Museu de Zoologia da Universidade de São Paulo, São Paulo
Franco ACS, Santos LN, Petry AC, García-Berthou E (2018) Abundance of invasive peacock bass increases with water residence time of reservoirs in southeastern Brazil. Hydrobiologia 817:155–166. https://doi.org/10.1007/s10750-017-3467-x
Freire CA, Amado EM, Souza LR, Veiga MPT, Vitule JRS, Souza MM, Prodocimo V (2008) Muscle water control in crustaceans and fishes as a function of habitat, osmoregulatory capacity, and degree of euryhalinity. Comp Biochem Physiol 149:435–446. https://doi.org/10.1016/J.CBPA.2008.02.003
Garcia AM, Vieira JP, Winemiller KO (2004) Grimm AM. Comparison of 1982–1983 and 1997–1998 El Niño effects on the shallow-water fish assemblage of the Patos Lagoon Estuary (Brazil). Estuaries 27:905–914
Garcia AM, Jaureguizar AJ, Protogino LC (2010) From fresh water to the slope: fish community ecology in the Rio de la Plata and the sea beyond. Lat Am J Aquat Res 38:81–94
Gutierre SMM, Vitule JRS, Freire CA, Prodocimo V (2014) Physiological tools to predict invasiveness and spread via estuarine bridges: tolerance of Brazilian native and worldwide introduced freshwater fishes to increased salinity. Mar Freshw Res 65:425–436. https://doi.org/10.1071/MF13161
Gutierre SMM, Schulte JM, Schofield PJ, Prodocimo V (2017) Osmorregulation and muscle water control in vitro facing salinity stress of the Amazon fish Oscar Astronotus ocellatus (Cichlidae). Mar Freshw Behav Physol 50(4):303–311. https://doi.org/10.1080/10236244.2017.1387480
Havel JE, Lee CE, Vander Zanden MJ (2005) Do reservoirs facilitate invasions into landscapes? Bioscience 55:515–525
Hoeinghaus DJ, Agostinho AA, Gomes LC, Pelicice FM et al (2009) Effects of river impoundment on ecosystem services of large Tropical rivers: Embodied energy and market value of artisanal fisheries. Conserv Biol 23:1222–1231
Jackson DA (1993) Stopping rules in principal component analysis: a comparison of heuristical and statiscal approaches. Ecology 74:2204–2214
Jackman S (2020) pscl: Classes and Methods for R Developed in the Political Science Computational Laboratory. United States Studies Centre, University of Sydney, Sydney, New South Wales, Australia. R package version 1.5.5, https://github.com/atahk/pscl/
Jaramillo-Villa U (2010) Efeito da Retificação de Rios sobre as Taxocenoses de Peixes: Estudo em Rios Costeiros de Mata Atlântica no Estado do Rio de Janeiro, Brasil. Master Dissertation, Rio de Janeiro: Universidade Federal do Rio de Janeiro
Johnson PTJ, Olden JD, Vander Zanden MJ (2008) Dam invaders: impoundments facilitate biological invasions into freshwaters. Front Ecol Environ 6:357–363
Kindt R, Coe R (2005) Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. World Agroforestry Centre (ICRAF), Nairobi. ISBN 92-9059-179-X
Kullander SO, Ferreira EJG (2006) A review of the South American cichlid genus Cichla, with descriptions of nine new species (Teleostei: Cichlidae). Ichthyol Explor Freshw 17(4):289–398
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129(2):271–280. https://doi.org/10.1007/s004420100716
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10(3):689–710
Magellan K, García-Berthou E (2015) Influences of size and sex on invasive species aggression and native species vulnerability: a case for modern regression techniques. Rev Fish Biol Fish 25(3):537–549
Marçal M, Brierley G, Lima R (2017) Using geomorphic understanding of catchment-scale process relationships to support the management of river futures: Macaé Basin, Brazil. Appl Geogr 84:23–41
Mendonça HS, Santos ACA, Martins MM, Araújo FG (2018) Size-related and seasonal changes in the diet of the non-native Cichla kelberi Kullander & Ferreira, 2006 in a lowland reservoir in the southeastern Brazil. Biota Neotrop 18(3):e20170493. https://doi.org/10.1590/10.1590/1676V0611VBNV2017V0493&
Menezes NA, Figueiredo JL (1980) Manual de Peixes Marinhos do Sudeste do Brasil. IV. Teleostei (3). Museu de Zoologia da Universidade de São Paulo, São Paulo
Menezes NA, Figueiredo JL (1985) Manual de Peixes Marinhos do Sudeste do Brasil. V. Teleostei (4). Museu de Zoologia da Universidade de São Paulo, São Paulo
Moyle PB, Light T (1996) Fish invasions in California: do abiotic factors determine success. Ecology 77:1666–1670
Myers GS (1937) Fresh-water fishes and West indian zoogeography. Rep Smithsonian Instn 92:339–364
Neves LM, Teixeira TP, Araújo FG (2010) Structure and dynamics of distinct fish assemblages in three reaches (upper, middle and lower) of an open tropical estuary in Brazil. Mar Ecol 32:115–131. https://doi.org/10.1111/j.1439-0485.2010.00407.x
Oksanen JF, Blanchet G, Friendly M, Kindt R et al 2019. vegan: Community Ecology Package. R package version 2.5-6. Available from: https://CRAN.R-project.org/package=vegan
Pelicice FM, Agostinho AA (2009) Fish fauna destruction after the introduction of a non-native predator (Cichla kelberi) in a Neotropical reservoir. Biol Invasions 11:1789–1801
Pelicice FM, Latini JD, Agostinho AA (2015) Fish fauna disassembly after the introduction of a voracious predator: main drivers and the role of the invader’s demography. Hydrobiologia 746:271–283. https://doi.org/10.1007/s10750-014-1911-8
Pelicice FM, Azevedo-Santos VM, Vitule JRS, Orsi ML et al (2017) Neotropical freshwater fishes imperilled by unsustainable policies. Fish Fish 18:1119–1133
Pelicice FM, Azevedo-Santos VM, Esguícero ALH, Agostinho AA, Arcifa MS (2018) Fish diversity in the cascade of reservoirs along the Paranapanema River, southeast Brazil. Neotrop Ichthyol 16(2):e170150
Peterson MS, Slack WT, Brown-Peterson N (2004) McDonald JL (2004) Reproduction in nonnative environments: establishment of Nile tilapia, Oreochromis niloticus, in Coastal Mississipi Watersheds. Copeia 4:842–849
Petesse ML, Petrere M Jr (2012) Tendency towards homogenization in fish assemblages in the cascade reservoir system of the Tietê river basin, Brazil. Ecol Eng 48:109–116
Poff NL, Olden JD, Merritt DM, Pepin DM (2007) Homogenization of regional river dynamics by dams and global biodiversity implications. PNAS 104(14):5732–5737
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ricciardi A, Hoopes MF, Marchetti MP, Lockwood JL (2013) Progress toward understanding the ecological impacts of nonnative species. Ecol Monog 83:263–282. https://doi.org/10.1890/13-0183.1
Schofield PJ, Peterson MS, Lowe MR, Brown-Peterson NJ, Slack WT (2011) Survival, growth and reproduction of nonindigenous Nile tilapia, Oreochromis niloticus (Linnaeus 1758). I. Physiological capabilities in various temperatures and salinities. Mar Freshw Res 62:439–449. https://doi.org/10.1071/MF10207
Simberloff D, Martin J-L, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, García-Berthou E, Pascal M, Pyšek P, Sousa R, Tabacchi E, Vilà M (2013) Impacts of biological invasions—what’s what and the way forward. Trends Ecol Evol 28:58–66
Sofiati A (2010) Macaé em quatro tempos. Herculano S, Correa HD (org) Oficina sobre Impactos Sociais, Ambientais e Urbanos das Atividades Petrolíferas: o caso de Macaé (RJ). UFF, Niterói, pp 130–148
Vitule JRS, Skóra F, Abilhoa V (2012) Homogenization of freshwater fish faunas after the elimination of a natural barrier by a dam in neotropics. Divers Distrib 18:111–120
Vitule JRS, Gazola da Silva FF, Bornatowski H, Abilhoa V (2013) Feeding ecology of fish in a coastal river of the Atlantic Rain Forest. Environ Biol Fish 96:1029–1044. https://doi.org/10.1007/S10641-012-0101-7
Winemiller KO (2001) Ecology of peacock cichlids (Cichla spp.) in Venezuela. J Aquaric Aquat Sci 9:93–112
Acknowledgements
The authors are grateful for the partnership made in the field work with Erica Pellegrini and Vagner dos Santos. We also thank students of the Laboratório de Ecologia de Peixes and Laboratório Integrado de Zoologia of the Universidade Federal do Rio de Janeiro for help in the samplings and laboratory work. The authors thank Arthur Bauer for the map and two anonymous reviewers for helpful comments. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. P. A. Catelani also thanks CAPES for the sandwich doctorate scholarship (CSF-PVE-S 88887.127845/2016-00). Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) provided research grants for F. M. Pelicice. E.G.B. was financially supported by the Spanish Ministry of Science and Innovation (projects CGL2016‐80820-R, PCIN-2016-168, and RED2018‐102571‐T) and the Government of Catalonia (ref. 2017 SGR 548). The comments and suggestions made by the Reviewers and the Editor helped to improve earlier versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Catelani, P.A., Petry, A.C., Pelicice, F.M. et al. When a freshwater invader meets the estuary: the peacock bass and fish assemblages in the São João River, Brazil. Biol Invasions 23, 167–179 (2021). https://doi.org/10.1007/s10530-020-02363-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-020-02363-w