Abstract
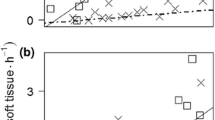
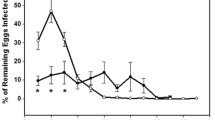
All-male populations of the freshwater prawn Macrobrachium rosenbergii were recently produced by a novel temporal RNA interference (RNAi)-based biotechnology for aquaculture purposes. This biotechnology opens the way to the wide use of all-male prawn populations as sustainable biocontrol agents against invading populations of freshwater snails, for which there is currently no environmentally friendly solution. Among the most damaging of the invasive freshwater snail species are the apple snails (Pomacea spp.), which inflict major damage on natural ecosystems and rice fields. The proposed use of all-male prawn populations as environmentally friendly biocontrol agents against invasive freshwater snails has several advantages: efficient predation by the prawns over a wide range of freshwater snails, the ready availability of the prawns, and the monosex non-reproductive nature of the biocontrol agents. Since the aquatic predators are strongly size selective, we quantified the predation rate as a function of body size of both predator and prey (M. rosenbergii and P. caniculata). Medium-sized and large prawns (~10–30 g) efficiently preyed small and medium-sized snails (up to 15 mm), while small prawns (up to 4 g) immediately and completely eradicated snail hatchlings. Medium-sized prawns (~22 g) exterminated a significant fraction of snail biomass within 24 h (up to 58% of their body mass) after being introduced into a tank of snails. A typical ‘climbing-to-the surface’ anti-predator behavior of the snails was recorded. The potential of all-male prawns as efficient biocontrol agents over hatchling and adult apple snails as part of an integrated pest management program is discussed. Our experiments set the stage for evaluating the ecological and economic implications of this generic solution for a wide variety of habitats.




Similar content being viewed by others
References
Adalla CB, Magsino EA, Joshi R, Sebastian L (2006) Understanding the golden apple snail (Pomacea canaliculata): biology and early initiatives to control the pest in the Philippines. Glob Adv Ecol Manag Gold Apple Snails: 199–213
Aflalo ED, Dandu RVSN, Verghese JT, Rao N, Samraj TYC, Ovadia O, Sagi A (2014) Neo-females production and all-male progeny of a cross between two Indian strains of prawn (Macrobrachium rosenbergii): population structure and growth performance under different harvest strategies. Aquaculture 428:7–15
Aizaki K, Yusa Y (2009) Field observations of the alarm response to crushed conspecifics in the freshwater snail Pomacea canaliculata: effects of habitat, vegetation, and body size. J Ethol 27:175–180
Barker GM (2002) Molluscs as crop pests. CABI publishing, New York
Bauer RT (2011) Amphidromy and migrations of freshwater shrimps. ii. Delivery of hatching larvae to the sea, return juvenile upstream migration, and human impacts. In: Asakura A (ed) New Frontiers in crustacean biology. Koninklijke Brill NV, Leiden
Boock MV, de Almeida Marques HL, Mallasen M, Barros HP, Moraes-Valenti P, Valenti WC (2016) Effects of prawn stocking density and feeding management on rice–prawn culture. Aquaculture 451:480–487
Burky AJ (1974) Growth and biomass production of an amphibious snail, Pomacea urceus (Müller), from the Venezuelan savannah. J Molluscan Stud 41:127–143
Burky AJ, Pacheco J, Pereyra E (1972) Temperature, water, and respiratory regimes of an amphibious snail, Pomacea urceus (Müller), from the Venezuelan savannah. Biol Bull 143:304–316
Carlsson N, Kestrup Å, Mårtensson M, Nyström P (2004a) Lethal and non-lethal effects of multiple indigenous predators on the invasive golden apple snail (Pomacea canaliculata). Freshw Biol 49:1269–1279
Carlsson NO, Brönmark C, Hansson L-A (2004b) Invading herbivory: the golden apple snail alters ecosystem functioning in Asian wetlands. Ecology 85:1575–1580
Carlsson NOL, Bronmark C, Hansson LA (2004c) Invading herbivory: the golden apple snail alters ecosystem functioning in Asian wetlands. Ecology 85:1575–1580
Charniaux-Cotton H (1954) Discovery in, an amphipod crustacean (Orchestia gammarella) of an endocrine gland responsible for the differentiation of primary and secondary male sex characteristics. Comptes rendus de l’Acad des Sci 239:780–782
Chung JS, Manor R, Sagi A (2011) Cloning of an insulin-like androgenic gland factor (IAG) from the blue crab, Callinectes sapidus: implications for eyestalk regulation of IAG expression. Gen Comp Endocrinol 173:4–10
Covich AP, Crowl TA, Alexander JE Jr, Vaughn CC (1994) Predator-avoidance responses in freshwater decapod-gastropod interactions mediated by chemical stimuli. J N Am Benthol Soc 13:283–290
EFSA (2014) EFSA panel on plant health (PLH), scientific opinion on the environmental risk assessment of the apple snail for the EU. EFSA J 12(4):3641, 97 pp
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Halwart M (1994) The golden apple snail Pomacea canaliculata in Asian rice farming systems: present impact and future threat. Int J Pest Manag 40:199–206
Harikrishnan M, Kurup BM (2001) Fishery of Macrobrachium rosenbergii (de Man) in the Vembanad lake and confluent rivers. Indian J Fish 48:189–198
Herrera FDA, Uribe ES, Ramirez LFB, Mora AG (1998) Critical thermal maxima and minima of Macrobrachium rosenbergii (Decapoda: Palaemonidae). J Therm Biol 23:381–385
Horgan FG, Stuart AM, Kudavidanage EP (2014) Impact of invasive apple snails on the functioning and services of natural and managed wetlands. Acta Oecologica Int J Ecol 54:90–100
Hosmer D, Lemeshow S, May S (1999) Applied survival analysis: regression modelling of time to event data. John Wiley & Sons, New York
Joshi RC, Sebastian LS (2006) Global advances in ecology and management of golden apple snails. Philippine Rice Research Institute (PhilRice), Philippines
Kwong K-L, Chan RK, Qiu J-W (2009) The potential of the invasive snail Pomacea canaliculata as a predator of various life-stages of five species of freshwater snails. Malacologia 51:343–356
Lach L, Cowie R (1999) The spread of the introduced freshwater apple snail Pomacea canaliculata(Lamarck)(Gastropoda: Ampullariidae) on Oahu, Hawaii. Bish Mus Occas Pap 58:66–71
Lee PG, Rodrick GE, Sodeman WA Jr, Blake NJ (1982) The giant Malaysian prawn, Macrobrachium rosenbergii, a potental predator for controlling the spread of schistosome vector snails in fish ponds. Aquaculture 28:293–301
Lezer Y, Aflalo ED, Manor R, Sharabi O, Abilevich LK, Sagi A (2015) On the safety of RNAi usage in aquaculture: the case of all-male prawn stocks generated through manipulation of the insulin-like androgenic gland hormone. Aquaculture 435:157–166
Lin DY, Wei L-J (1989) The robust inference for the Cox proportional hazards model. J Am Stat Assoc 84:1074–1078
Litsinger J, Estano DB (1993) Management of the golden apple snail Pomacea canaliculata (Lamarck) in rice. Crop Prot 12:363–370
Manor R, Aflalo ED, Segall C, Weil S, Azulay D, Ventura T, Sagi A (2004) Androgenic gland implantation promotes growth and inhibits vitellogenesis in Cherax quadricarinatus females held in individual compartments. Invertebr Reprod Dev 45:151–159
Manor R, Weil S, Oren S, Glazer L, Aflalo ED, Ventura T, Chalifa-Caspi V, Lapidot M, Sagi A (2007) Insulin and gender: an insulin-like gene expressed exclusively in the androgenic gland of the male crayfish. Gen Comp Endocrinol 150:326–336
Mareddy VR, Rosen O, Thaggard HB, Manor R, Kuballa AV, Aflalo ED, Sagi A, Paterson B, Elizur A (2011) Isolation and characterization of the complete cDNA sequence encoding a putative insulin-like peptide from the androgenic gland of Penaeus monodon. Aquaculture 318:364–370
Naylor R (1996) Invasions in agriculture: assessing the cost of the golden apple snail in Asia. Ambio 25:443–448
Pain T (1960) Pomacea (Ampullariidae) of the Amazon river system. J Conchol 24:421–432
Perera G (1996) Apple snails in the aquarium: Ampullariids-their identification, care, and breeding. TFH Publications, New Jersey
Prashad B (1925) Anatomy of the common Indian apple-snail, Pila globosa. Zoological Survey of India, India
Roberts JK, Kuris AM (1990) Predation and control of laboratory populations of the snail Biomphalaria glabrata by the freshwater prawn Macrobrachium rosenbergii. Ann Trop Med Parasitol 84:401–412
Roll U, Dayan T, Simberloff D, Mienis HK (2009) Non-indigenous land and freshwater gastropods in Israel. Biol Invasions 11:1963–1972
Sagi A, Cohen D (1990) Growth, maturation and progeny of sex-reversed Macrobrachium rosenbergii males. World Aquac 21:87–90
Sagi A, Ra’Anan Z, Cohen D, Wax Y (1986) Production of Macrobrachium rosenbergii in monosex populations: yield characteristics under intensive monoculture conditions in cages. Aquaculture 51:265–275
Sagi A, Snir E, Khalaila I (1997) Sexual differentiation in decapod crustaceans: role of the androgenic gland. Invertebr Reprod Dev 31:55–61
Savaya-Alkalay A, Rosen O, Sokolow SH, Faye YP, Faye DS, Aflalo ED, Jouanard N, Zilberg D, Huttinger E, Sagi A (2014) The prawn Macrobrachium vollenhovenii in the Senegal river basin: towards sustainable restocking of all-male populations for biological control of schistosomiasis. PLoS Negl Trop Dis 8(8):e3060
Shpak N, Manor R, Aflalo ED, Sagi A (2017) Three generations of cultured prawn without W chromosome. Aquaculture 467(C):41–48
Simberloff D, Stiling P (1996) How risky is biological control? Ecology 77:1965–1974
Sokolow SH, Lafferty KD, Kuris AM (2013) Regulation of laboratory populations of snails (Biomphalaria and Bulinus spp.) by river prawns, Macrobrachium spp. (Decapoda, Palaemonidae): implications for control of schistosomiasis. Acta Trop 132C:64–67
Sokolow SH, Wood CL, Jones IJ, Swartz SJ, Lopez M, Hsieh MH, Lafferty KD, Kuris AM, Rickards C, De Leo GA (2016) Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Negl Trop Dis 10(7):e0004794
Sokolow SH, Jones IJ, Jocque M, La D, Cords O, Knight A, Lund A, Wood CL, Lafferty KD, Hoover CM (2017) Nearly 400 million people are at higher risk of schistosomiasis because dams block the migration of snail-eating river prawns. Phil Trans R Soc B 372:20160127
Taketomi Y, Murata M, Miyawaki M (1990) Androgenic gland and secondary sexual characters in the crayfish Procambarus clarkii. J Crustac Biol 10:492–497
Torres MV, Giri F, Williner V (2012) Size selective predation on an invasive bivalve, Limnoperna fortunei (Mytilidae), by a freshwater crab, Zilchiopsis collastinensis (Trichodactylidae). J Crustac Biol 32:698–710
Touir A (1977) New data concerning sexual endocrinology of hermaphroditic and gonochoristic crustacea decapoda natantia. 2. Maintenance of gonia and evolution of gametogenesis invivo and invitro. Comptes Rendus Hebd Des Seances De L Acad Des Sci Serie D 284:2515–2518
Ueshima E, Yusa Y (2015) Antipredator behaviour in response to single or combined predator cues in the apple snail Pomacea canaliculata. J Molluscan Stud 81(1):51–57
van Lenteren J, Babendreier D, Bigler F, Burgio G, Hokkanen H, Kuske S, Loomans A, Menzler-Hokkanen I, van Rijn P, Thomas M (2003) Environmental risk assessment of exotic natural enemies used in inundative biological control. Biocontrol 48:3–38
Vázquez-Islas G, Garza-Torres R, Guerrero-Tortolero DA, Campos-Ramos R (2014) Histology of the androgenic gland and expression of the insulin-like androgenic gland hormone precursor gene in the genital organ of Pacific white shrimp Litopenaeus vannamei. J Crustac Biol 34:293–299
Ventura T, Sagi A (2012) The insulin-like androgenic gland hormone in crustaceans: from a single gene silencing to a wide array of sexual manipulation-based biotechnologies. Biotechnol Adv 30:1543–1550
Ventura T, Manor R, Aflalo ED, Weil S, Raviv S, Glazer L, Sagi A (2009) Temporal silencing of an androgenic gland-specific insulin-like gene affecting phenotypical gender differences and spermatogenesis. Endocrinology 150:1278–1286
Ventura T, Manor R, Aflalo ED, Weil S, Rosen O, Sagi A (2012) Timing sexual differentiation: full functional sex reversal achieved through silencing of a single insulin-like gene in the prawn, Macrobrachium rosenbergii. Biol Reprod 86:6
Wada T (1997) Introduction of the apple snail Pomacea canaliculata and its impact on rice agriculture. In: Proceedings of the international workshop on biological invasions of ecosystems by pests and beneficial organisms, pp 170–180
Yamanishi Y, Yoshida K, Fujimori N, Yusa Y (2012) Predator-driven biotic resistance and propagule pressure regulate the invasive apple snail Pomacea canaliculata in Japan. Biol Invasions 14:1343–1352
Yoshie H, Yusa Y (2011) Indirect interactions in a rice ecosystem: density dependence and the interplay between consumptive and non-consumptive effects of predators. Freshw Biol 56:302–310
Yusa Y, Wada T (1999) Impact of the introduction of apple snails and their control in Japan. Naga ICLARM Q 22:9–13
Yusa Y, Sugiura N, Wada T (2006) Predatory potential of freshwater animals on an invasive agricultural pest, the apple snail Pomacea canaliculata (Gastropoda: ampullariidae), in southern Japan. Biol Invasions 8:137–147
Acknowledgements
We would like to thank Mr Yossi Savaia, Mr Anton Fennec, Mr Dan Davidi, Kavra Ltd. and courtesy of Tiran Shipping group Ltd. through their subcontractor Mr. Ran Epshtein, Colors Ltd. for animal supply. In addition we thank Mr Shalev Goldferb, Mrs Nurit Levi, Mr Ran Marziano, Mr Shahar Sagie and Mr Yishai Shuchalter for animal maintenance at BGU.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MP4 4025 kb)
Supplementary material 3 (MP4 7490 kb)
Rights and permissions
About this article
Cite this article
Savaya-Alkalay, A., Ovadia, O., Barki, A. et al. Size-selective predation by all-male prawns: implications for sustainable biocontrol of snail invasions. Biol Invasions 20, 137–149 (2018). https://doi.org/10.1007/s10530-017-1522-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1522-1